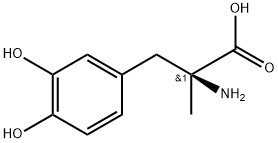
Methyldopa synthesis
- Product Name:Methyldopa
- CAS Number:555-30-6
- Molecular formula:C10H13NO4
- Molecular Weight:211.22
![Propanedioic acid, 2-[(3,4-dimethoxyphenyl)methyl]-2-methyl-, 1,3-dimethyl ester](/CAS/GIF/5846-22-0.gif)
5846-22-0
0 suppliers
inquiry

555-30-6
370 suppliers
$45.00/1g
Yield:-
Steps:
Multi-step reaction with 3 steps
1: α-chymotrypsin / dimethylsulfoxide; H2O / 22 - 25 °C / potassium phosphate ( pH 7.0); with pig liver esterase the reaction rate is higher but the e.e. is lower
2: 1.) acyl azide formation, 2.) Curtius rearrangement
3: aq. HBr (48percent)
References:
Bjoerkling, Fredrik;Boutelje, John;Gatenbeck, Sten;Hult, Karl;Norin, Torbjoern [Tetrahedron Letters,1985,vol. 26,# 40,p. 4957 - 4958]

21852-32-4
111 suppliers
$50.00/250mg

555-30-6
370 suppliers
$45.00/1g

1395919-79-5
0 suppliers
inquiry

555-30-6
370 suppliers
$45.00/1g
![4-Imidazolidinone, 1-benzoyl-5-[(3,4-dimethoxyphenyl)methyl]-2-(1,1-dimethylethyl)-3,5-dimethyl-, (2S,5S)-](/CAS/20210305/GIF/98262-54-5.gif)
98262-54-5
0 suppliers
inquiry

555-30-6
370 suppliers
$45.00/1g
![Benzenemethanamine, N-[(3,4-dimethoxyphenyl)methylene]-](/CAS/20210305/GIF/33859-00-6.gif)
33859-00-6
0 suppliers
inquiry

555-30-6
370 suppliers
$45.00/1g