| Identification | More | [Name]
Ethylenzene | [CAS]
100-41-4 | [Synonyms]
AKOS BBS-00004368
DIETHYL ETHER
DIETHYL ETHER 300
DIETHYL ETHER 5000
DIETHYL OXIDE
ET2O
ETHER
ETHOXYETHANE
ETHYLBENZENE
ethylenzene
ETHYL ETHER
ETHYL ETHER 1000
ETHYL OXIDE
PHENYLETHANE
a.-Methyltoluene
Aethylbenzol
alpha-Methyltoluene
Benzene, ethyl-
benzene,ethyl-
EB | [EINECS(EC#)]
200-467-2 | [Molecular Formula]
C2H2 | [MDL Number]
MFCD00011647 | [Molecular Weight]
26.04 | [MOL File]
100-41-4.mol |
| Chemical Properties | Back Directory | [Appearance]
Ethylbenzene is a colorless liquid. Pungent
aromatic odor. The Odor Threshold is 0.092�0.60 ppm | [Melting point ]
−95 °C(lit.)
| [Boiling point ]
136 °C(lit.) | [density ]
0.867 g/mL at 25 °C(lit.)
| [vapor density ]
3.7 (vs air)
| [vapor pressure ]
28.69 psi ( 55 °C)
| [refractive index ]
n20/D 1.495(lit.)
| [Fp ]
72 °F
| [storage temp. ]
0-6°C | [solubility ]
0.2g/l | [form ]
Liquid | [color ]
Colorless | [Odor]
Aromatic. | [Relative polarity]
0.117 | [Stability:]
Stable. Incompatible with oxidizing agents. Flammable. | [explosive limit]
1.0-7.8%(V) | [Odor Threshold]
0.17ppm | [Water Solubility ]
0.0206 g/100 mL | [FreezingPoint ]
-95℃ | [Merck ]
14,3765 | [BRN ]
1901871 | [Henry's Law Constant]
13.9(x 10-3 atm?m3/mol) at 45.00 °C, 15.1 at 50.00 °C, 17.1 at 55.00 °C, 20.1 at 60.00 °C, 20.9 at 65.00 °C, 22.7 at
70.00 °C, 34.3 at 80.00 °C (static headspace-GC, Park et al., 2004) | [Dielectric constant]
2.5(20℃) | [Exposure limits]
TLV-TWA 100 ppm (~433 mg/m3) (ACGIH,
NIOSH, MSHA, and OSHA); STEL 125 ppm
(541 mg/m3) (ACGIH); IDLH 2000 ppm
(NIOSH). | [InChIKey]
YNQLUTRBYVCPMQ-UHFFFAOYSA-N | [LogP]
3.03-3.6 at 20℃ | [CAS DataBase Reference]
100-41-4(CAS DataBase Reference) | [IARC]
2B (Vol. 77) 2000 | [NIST Chemistry Reference]
Ethylbenzene(100-41-4) | [EPA Substance Registry System]
100-41-4(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
F+,Xn,Xi,F,T | [Risk Statements ]
R12:Extremely Flammable.
R19:May form explosive peroxides.
R22:Harmful if swallowed.
R66:Repeated exposure may cause skin dryness or cracking.
R67:Vapors may cause drowsiness and dizziness.
R20:Harmful by inhalation.
R11:Highly Flammable.
R48/20/22:Harmful: danger of serious damage to health by prolonged exposure through inhalation and if swallowed .
R40:Limited evidence of a carcinogenic effect.
R38:Irritating to the skin.
R36/37/38:Irritating to eyes, respiratory system and skin .
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed .
R45:May cause cancer.
R39/23/24/25:Toxic: danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed .
R23/25:Toxic by inhalation and if swallowed . | [Safety Statements ]
S9:Keep container in a well-ventilated place .
S16:Keep away from sources of ignition-No smoking .
S29:Do not empty into drains .
S33:Take precautionary measures against static discharges .
S24/25:Avoid contact with skin and eyes .
S36/37:Wear suitable protective clothing and gloves .
S36:Wear suitable protective clothing .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S23:Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer) .
S53:Avoid exposure-obtain special instruction before use .
S7:Keep container tightly closed .
S24:Avoid contact with skin . | [RIDADR ]
UN 1175 3/PG 2
| [WGK Germany ]
1
| [RTECS ]
KI5775000
| [F ]
10 | [Autoignition Temperature]
810 °F | [TSCA ]
Yes | [HazardClass ]
3 | [PackingGroup ]
II | [HS Code ]
29026000 | [Safety Profile]
Moderately toxic by
ingestion and intraperitoneal routes. Mildly
toxic by inhalation and skin contact. An
experimental teratogen. Other experimental
reproductive effects. Human systemic
effects by inhalation: eye, sleep, and
pulmonary changes. An eye and skin irritant.
Human mutation data reported. The liquid
is an irritant to the skin and mucous
membranes. A concentration of 0.1% of the
vapor in air is an irritant to human eyes, and
a concentration of 0.2% is extremely
irritating at first, then causes dizziness,
irritation of the nose and throat, and a sense
of constriction in the chest. Exposure of
guinea pigs to 1% concentration has been
reported as causing ataxia, loss of
consciousness, tremor of the extremities,
and finally death through respiratory failure.
The pathological findings were congestion
of the brain and lungs with edema. A very dangerous fire and explosion hazard when exposed to heat or flame; can
react vigorously with oxidizing materials. To
fight fire, use foam, CO2, dry chemical.
Emitted from modern budding materials
(CENEAR 69,22,91). When heated to
decomposition it emits acrid smoke and
irritating fumes.
| [Hazardous Substances Data]
100-41-4(Hazardous Substances Data) | [Toxicity]
LD50 orally in rats: 5.46 g/kg (Smyth) | [IDLA]
800 ppm [10% LEL] |
| Hazard Information | Back Directory | [General Description]
A clear colorless liquid with an aromatic odor. Flash point 59°F. Less dense than water (at 7.2 lb/gal) and insoluble in water. Hence floats on water. Vapors heavier than air. Used as a solvent and to make other chemicals. | [Reactivity Profile]
ETHYLBENZENE(100-41-4) can react vigorously with strong oxidizing materials . | [Air & Water Reactions]
Highly flammable. Insoluble in water. | [Hazard]
Toxic by ingestion, inhalation, and skin
absorption; irritant to skin and eyes. Flammable,
dangerous fire risk. Possible carcinogen. | [Health Hazard]
Inhalation may cause irritation of nose, dizziness, depression. Moderate irritation of eye with corneal injury possible. Irritates skin and may cause blisters. | [Potential Exposure]
Ethyl benzene is used in styrene manufacture and in synthesis of p-nitroacetophenone; in the
manufacture of cellulose acetate, and synthetic rubber. It is
also used as a solvent or diluent; and as a component of
automotive and aviation gasoline. Significant quantities of
EB are present in mixed xylenes. These are used as dilatants in the paint industry, in agricultural sprays for insecticides and in gasoline blends (which may contain as much
as 20% EB). In light of the large quantities of EB produced
and the diversity of products in which it is found, there
may exist environmental sources for ethylbenzene, e.g.,
vaporization during solvent use; pyrolysis of gasoline and
emitted vapors at filling stations. Groups of individuals
who are exposed to EB to the greatest extent and could represent potential pools for the expression of EB toxicity
include: (1) individuals in commercial situations where
petroleum products or by-products are manufactured e.g.,
rubber or plastics industry); (2) individuals residing in areas
with high atmospheric smog generated by motor vehicle
emissions | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit. | [Shipping]
UN1175 Ethylbenzene, Hazard Class: 3; Labels:
3-Flammable liquid | [Incompatibilities]
Vapors may form explosive mixture with
air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,oxoacids, and epoxides. Attacks plastics and rubber. May
accumulate static electrical charges, and may cause ignition
of its vapors. | [Description]
Ethylbenzene is a colorless liquid with a pungent aromaticodor. The odor threshold is 0.092- 0.60 ppm.Molecular weight = 106.18; Specific gravity (H2O:1) =0.87; Boiling point = 136℃; Freezing/Meltingpoint = - 95℃; Vapor pressure = 7% mmHg at 20℃;Flash point = 12.8℃; Autoignition temperature = 432℃.Explosive limits: LEL = 0.8%; UEL = 6.7%. HazardIdentification (based on NFPA-704 M Rating System):Health 2, Flammability 3, Reactivity 0. Practically insolublein water; solubility = 0.7%. | [Waste Disposal]
Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator
equipped with an afterburner and scrubber. All federal, state,
and local environmental regulations must be observed | [Physical properties]
Clear, colorless liquid with a sweet, gasoline-like odor. At 40 °C, the average odor threshold
concentration and the lowest concentration at which an odor was detected were 550 and 150 μg/L,
respectively. Similarly, at 25 °C, the average taste threshold concentration and the lowest
concentration at which a taste was detected were 780 and 390 μg/L, respectively (Young et al.,
1996). The average least detectable odor threshold concentrations in water at 60 °C and in air at 40
°C were 2.4 and 72 μg/L, respectively (Alexander et al., 1982). Cometto-Mu?iz and Cain (1994)
reported an average nasal pungency threshold concentration of 10,100 ppmv. | [Occurrence]
Has apparently not been reported to occur in nature. | [Definition]
ethylbenzene: A colourless flammableliquid, C6H5C2H5; r.d. 0.867;m.p. –95°C; b.p. 136°C. It is madefrom ethene and ethybenzene by aFriedel–Crafts reaction and is usedin making phenylethene (for polystyrene). | [Production Methods]
Ethylbenzene is produced by alkylation of benzene with ethylene, except for a very small fraction that is recovered from mixed C8 aromatics by superfractionation. The reaction takes place on acidic catalysts and can be carried out either in the liquid or vapor phase. | [Synthesis Reference(s)]
Chemistry Letters, 12, p. 909, 1983
Journal of the American Chemical Society, 85, p. 2768, 1963 DOI: 10.1021/ja00901a021
Tetrahedron Letters, 11, p. 4401, 1970 | [Flammability and Explosibility]
Highlyflammable | [Chemical Reactivity]
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. | [Source]
Detected in distilled water-soluble fractions of 87 octane gasoline (2.38 mg/L), 94 octane
gasoline (7.42 mg/L), Gasohol (3.54 mg/L), No. 2 fuel oil (0.21 mg/L), jet fuel A (0.41 mg/L),
diesel fuel (0.17 mg/L), military jet fuel JP-4 (1.57 mg/L) (Potter, 1996), new motor oil (0.15 to
0.17 μg/L), and used motor oil (117 to 124 μg/L) (Chen et al., 1994). The average volume percent
and estimated mole fraction in American Petroleum Institute PS-6 gasoline are 1.570 and 0.017,
respectively (Poulsen et al., 1992). Diesel fuel obtained from a service station in Schlieren,
Switzerland contained ethylbenzene at a concentration of 690 mg/L (Schluep et al., 2001).
Kaplan et al. (1996) determined ethylbenzene concentrations in four different grades of
gasolines. Average ethylbenzene concentrations were 9.1 g/L in regular unleaded gasoline, 8.0 g/L
in leaded gasoline, 9.3 g/L in unleaded plus gasoline, and 10.1 g/L in Super unleaded gasoline.
Thomas and Delfino (1991) equilibrated contaminant-free groundwater collected from
Gainesville, FL with individual fractions of three individual petroleum products at 24–25 °C for
24 h. The aqueous phase was analyzed for organic compounds via U.S. EPA approved test method
602. Average ethylbenzene concentrations reported in water-soluble fractions of unleaded
gasoline, kerosene, and diesel fuel were 2.025, 0.314, and 0.104 mg/L, respectively. When the
authors analyzed the aqueous-phase via U.S. EPA approved test method 610, average
ethylbenzene concentrations in water-soluble fractions of unleaded gasoline, kerosene, and diesel
fuel were lower, i.e., 1.423, 0.171, and 0.079 mg/L, respectively.
Schauer et al. (1999) reported ethylbenzene in a diesel-powered medium-duty truck exhaust at
an emission rate of 470 μg/km. California Phase II reformulated gasoline contained ethylbenzene
at a concentration of 12,800 mg/kg. Gas-phase tailpipe emission rates from gasoline-powered
automobiles with and without catalytic converters were 4.18 and 434.0 mg/km, respectively
(Schauer et al., 2002).
Detected in 1-yr aged coal tar film and bulk coal tar at concentrations of 350 and 2,100 mg/kg,
respectively (Nelson et al., 1996). A high-temperature coal tar contained ethylbenzene at an
average concentration of 0.02 wt % (McNeil, 1983).
Identified as one of 140 volatile constituents in used soybean oils collected from a processing
plant that fried various beef, chicken, and veal products (Takeoka et al., 1996).
Schauer et al. (2001) measured organic compound emission rates for volatile organic
compounds, gas-phase semi-volatile organic compounds, and particle-phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The gas-phase emission
rate of ethylbenzene was 22.9 mg/kg of pine burned. Emission rates of ethylbenzene were not
measured during the combustion of oak and eucalyptus. | [Environmental Fate]
Biological. Phenylacetic acid was reported to be the biooxidation product of ethylbenzene by Nocardia sp. in soil using n-hexadecane or n-octadecane as the substrate. In addition,
Methylosinus trichosporium OB3b was reported to metabolize ethylbenzene to 2- and 3-
hydroxybenzaldehyde with methane as the substrate (Keck et al., 1989). Ethylbenzene was
oxidized by a strain of Micrococcus cerificans to phenylacetic acid (Pitter and Chudoba, 1990). A
culture of Nocardia tartaricans ATCC 31190, growing in a hexadecane medium, oxidized
ethylbenzene to 1-phenethanol, which oxidized to acetophenone (Cox and Goldsmith, 1979).
When ethylbenzene (5 mg/L) was statically incubated in the dark at 25 °C with yeast extract and
settled domestic wastewater inoculum, complete biodegradation with rapid acclimation was
observed after 7 d. At a concentration of 10 mg/L, significant degradation occurred with gradual
adaptation. Percent losses of 69, 78, 87, and 100 were obtained after 7, 14, 21, and 28-d incubation
periods, respectively (Tabak et al., 1981). Olsen and Davis (1990) reported a first-order
degradation rate constant of 0.07/yr and a half-life of 37 d.
Surface Water. The evaporation half-life of ethylbenzene in surface water (1 m depth) at 25 °C
is estimated to be from 5 to 6 h (Mackay and Leinonen, 1975). Estimated half-lives of
ethylbenzene (3.3 μg/L) from an experimental marine mesocosm during the spring (8–16 °C),
summer (20–22 °C), and winter (3–7 °C) were 20, 2.1, and 13 d, respectively (Wakeham et al.,
1983).
Photolytic. Irradiation of ethylbenzene (λ <2537 ?) at low temperatures will form hydrogen,
styrene, and free radicals (Calvert and Pitts, 1966).
Chemical/Physical. Complete combustion in air yields carbon dioxide and water vapor.
Ethylbenzene will not hydrolyze in water (Kollig, 1993). | [Metabolism]
The main oxidation of ethyl benzene occurs at the activated α-methylene group to yield methylphenylcarbinol which is also the precursor of hippuric and mandelic acids. Both optical isomers of methylphenylcarbinol are formed, probably in equal amounts, and these have been isolated from the urine of rabbits as the corresponding glucuronides. The two optical forms of mandelic acid have also been found (Williams, 1959). | [storage]
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with this chemical you should be trainedon its proper handling and storage. Before entering confinedspace where this chemical may be present, check to makesure that an explosive concentration does not exist. Protectagainst physical damage. Outside or detached storage ispreferable. Inside storage should be in a standard flammableliquids storage room or cabinet. Isolate from acute firehazards and oxidizing agents. Store in tightly closed containers in a cool, well-ventilated area away from heat.Sources of ignition, such as smoking and open flames, areprohibited where this chemical is used, handled, or stored ina manner that could create a potential fire or explosion hazard. Metal containers involving the transfer of 5 gallons ormore of this chemical should be grounded and bonded.Drums must be equipped with self-closing valves, pressurevacuum bungs, and flame arresters. Use only nonsparkingtools and equipment, especially when opening and closingcontainers of this chemical. | [Toxicity evaluation]
Ethylbenzene acts by a variety of toxic mechanisms in affected
tissues/organs. Acute CNS depressive or narcotic effects occur
nonspecifically and likely result from unmetabolized ethylbenzene’s
disruption of neuronal membranes. Ototoxicity
also appears to result from unmetabolized ethylbenzene
damage to hair cells of the cochlea. Both of these toxicities are
relevant to humans. Other ethylbenzene toxicities appear to be
linked to metabolism or metabolites, and hence, differences are
expected between laboratory animals and humans. Induction
of cytochrome P450E1 is postulated to contribute to liver
changes in mice and kidney changes in rats. Rat kidney toxicity
is demonstrated to be associated with alpha-2u-globulin
nephropathy and exacerbation of chronic progressive
nephropathy, conditions that are not relevant to human health.
Lung toxicity is postulated to arise from mouse specific lung
enzyme (cytochrome P450F2) metabolism to cytotoxic
metabolite(s) and associated chronic cell proliferation in lung
target cells. |
| Questions And Answer | Back Directory | [Chemical Properties]
Ethylbenzene is a colorless, volatile, highly flammable liquid having a gasoline-like odor. Less dense than water and insoluble in water. Hence floats on water. Vapors are heavier than air. Ethylbenzene is a member of the family of chemicals called alkylbenzenes. These are aromatic compounds containing a benzene substituted at one or more positions. It is found in natural products such as coal tar and petroleum and is also found in manufactured products such as inks, insecticides, and paints. Ethylbenzene is used primarily to make another chemical, styrene. Other uses include as a solvent, in fuels, and to make other chemicals. It is manufactured commercially from benzene and ethylene. | [Uses]
Ethylbenzene is used primarily to make styrene monomer (SM) [1]counting greater than 99%. At less than 1%, it is used as a solvent[2], in fuels[3], or as a starting material[4] to make other chemicals.
[1] Ethylbenzene is mainly used in the manufacture of styrene, which is the raw material of producing styrene-based polymers that are widely used in products such as packaging, kitchen utensils and electronic equipment housing.2
[2] Solvents in a variety of Industries
Which industries
How it is used
Machinery Mfg. and Repair
Solvents
Rubber Manufacture
Solvents
Paint Manufacture
Hydrocarbon Solvents
Wood Stains and Varnishes
Varnish Solvent
Electroplating
Vapors Degreasing Solvents
[3] Ethyl benzene (C6H5C2H5) is the smallest aromatic hydrocarbon molecule with C C key chain structure, and the C C single bond adjacent to benzene ring is far more weaker than those of other chemical bonds thus it is the most easily broken key. It is the characteristic structure of single chain alternative fuels such as n-propylbenzene n-butylbenzene in diesel oil and aviation kerosene.
[4] Ethylbenzene may be used as a starting material to synthesize: (1) Acetophenone via selection oxidation in the presence of potassium dichromate supported on neutral alumina and using air as the oxidizing agent. (2) Styrene via dehydrogenation over nanodiamonds in an oxygen-lean environment.
| [Preparation]
Preparation of Ethylbenzene from Acetophenone.
Principle: The carbonyl function can be completely reduced to methylene under acidic, basic or neutral conditions. Amongst these the most common methods are Clemmensen's reduction, Wolf Kishner reduction and Mozingo reduction. The reduction of carbonyl group using hydrazine in basic medium using KOH is called as Wolf-Kishner reduction.
Reaction:
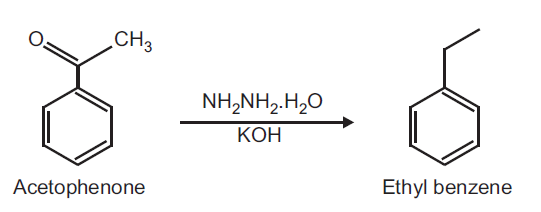
Procedure: Place 0.5 ml acetophenone, 5.0 ml ethylene glycol and 1.0 ml of 90% hydrazine hydrate solution and 1.1 g KOH pellets in a round bottom flask fitted with water condenser. Warm the reaction mixture of boiling water bath till KOH dissolves and then reflux for 1 h on wire gauze. Distill using Hickmann head till the temperature is 175oC. Keep the distillate and again reflux for 2 h. Cool this and extract twice with 10 ml ether. Combine the ether extracts with the distillate removed earlier and dry over sodium sulphate, decant and evaporate the ether.
| [Ethylbenzene Production]
Ethylbenzene is currently used on a large scale industrially for the production of styrene monomer. Ethylbenzene may be produced by a number of chemical processes but one process which has achieved a significant degree of commercial success is the alkylation of benzene with ethylene in the presence of a solid, acidic zeolite catalyst.
A preferred catalyst includes the synthetic zeolite identified in this specification as MCM-22.In the production of ethylbenzene by this process; ethylene is used as the alkylating agent and is reacted with benzene in the presence of the catalyst at certain temperatures.
Production of ethylbenzene involves the liquid-phase reaction of ethylene with benzene
C2H4 + C6H6 C8H10
Undesirable reaction occurred by the formation of Di-ethyl benzene from reaction of ethylbenzene with ethylene.
C8H10 + C2H4 C10H14
A third reaction also occurs, in which Di-ethyl benzene reacts with benzene to form ethylbenzene.
C10H14 + C6H6 2C8H10 | [Health Effects]
Ethylbenzene has low acute and chronic toxicity for humans. It is toxic to the central nervous system and is an irritant of mucous membranes and the eyes. Ethylbenzene exposure might be associated with hearing loss, neurobehavioral function impairment, and imbalance of neurotransmitters, and it is an inducer of liver microsomal enzymes. The toxicity is stronger along with the rise of exposure volume, see the table below:
Exposure Volume in air
Health effects
200 ppm
Ethylbenzene vapor has a transient irritant effect on human eyes
1000 ppm
On the first exposure it is very irritating and causes tearing, but tolerance rapidly develops.
2000 ppm
Eye irritation and lacrimation are immediate and severe.
2,000-5,000 ppm
Human exposures of ethylbenzene are associated with dizziness and vertigo
5000 ppm
It causes intolerable irritation of the eyes and nose
Carcinogenicity
CLASSIFICATION: D; not classifiable as to human carcinogenicity.
BASIS FOR CLASSIFICATION: nonclassifiable due to lack of animal bioassays and human studies.
|
|
|