| Identification | More | [Name]
HRP 2000 | [CAS]
126784-99-4 | [Synonyms]
VA 2914
EllaOne
RU 44675
CBD 2914
HRP 2000
Ulipristal
Ulipristal,Ella
Ulipristal Actate
Ulipristal acetate
Uliprisnil Acetate
Ulipristal acetate/CDB2914
CDB2914(Uliprisnil acetate)
Uliprisnil acetate(CDB2914)
17α-Acetoxy-11β-(4-dimethylaminophenyl)-19-norpregna-4,9-dien-3,20-dione
17a-Acetoxy-11b-[4-N,N-diMethylaMinophenyl]-19-norpregna-4,9-diene-3,20-dione
17alpha-Acetoxy-11beta-[4-(diMethylaMino)phenyl]-19-norpregna-4,9-diene-3,20-dione
(11β)-17-(Acetyloxy)-11-[4-(diMethylaMino)phenyl]-19-norpregna-4,9-diene-3,20-dione
(11b)-17-(Acetyloxy)-11-[4-(dimethylamino)phenyl]-19-norpregna-4,9-diene-3,20-dione
19-Norpregna-4,9-diene-3,20-dione,17-(acetyloxy)-11-[4-(diMethylaMino)phenyl]-, (11b)- | [EINECS(EC#)]
682-170-1 | [Molecular Formula]
C28H35NO3 | [MDL Number]
MFCD00899035 | [MOL File]
126784-99-4.mol | [Molecular Weight]
433.582 |
| Chemical Properties | Back Directory | [Melting point ]
183-185 ºC | [Boiling point ]
640.1±55.0 °C(Predicted) | [density ]
1.19 | [storage temp. ]
-20°C | [solubility ]
Chloroform, Ethyl Acetate, Methanol | [form ]
powder | [pka]
5.49±0.24(Predicted) | [color ]
white to beige | [optical activity]
[α]/D +165 to +185°, c = 1 in dichloromethane |
| Hazard Information | Back Directory | [Chemical Properties]
Pale Yellow Solid | [Usage]
Labelled Ulipristal | [Usage]
Ulipristal acetate is a selective progesterone receptor modulator | [Usage]
Α selective labelled progesterone receptor modulator | [Originator]
Research Triangle Institute (US) | [Uses]
Labelled Ulipristal | [Uses]
Ulipristal acetate is a selective progesterone receptor modulator | [Uses]
Α selective labelled progesterone receptor modulator | [Definition]
ChEBI: A 20-oxo steroid obtained by acetylation of the 17-hydroxy group of (11beta,17alpha)-17-acetyl-11-[4-(dimethylamino)phenyl]-3-oxoestra-4,9-dien-17-ol (ulipristal). A selective progesterone receptor modulator, which is empl
yed as an emergency contraceptive. | [Brand name]
ellaOne | [Biochem/physiol Actions]
Ulipristal acetate is a selective progesterone receptor modulator (SPRM) with tissue-selective partial antagonist activity. It has clinical use both as an emergency contraceptive and as a treatment for uterine fibroids. Ulipristal acetate acts as a partial antagonist on the hypothalamic–pituitary–ovarian axis to inhibit or delay ovulation without affecting human embryo implantation. As a progesterone antagonist, Ulipristal acetate selectively suppresses neo-vascularization, cell proliferation, and survival in uterine fibroid cells, but not in normal myometrial cells. | [Clinical Use]
Ulipristal acetate, a selective progesterone receptor modulator
(SPRM), was developed at the Research Triangle Institute. In
2009, HRA Pharma received FDA approval for emergency contraception
within 120 h (5 days) of unprotected sexual intercourse or contraceptive failure. Ulipristal acetate is a well-known steroid
that possesses antiprogestational and antiglucocorticoid activity.
It is the first SPRM that was specifically designed as an oral
emergency contraceptive. Unlike earlier levonorgestrel-based
emergency contraceptives, this SPRM drug maintains efficacy for
5 days after unprotected intercourse while having safety profile
comparable to levonorgestrel. | [Synthesis]
Recently, an industrial scale route
was published and is described in the scheme.89 Alkylation of
commercially available 3-(ethylenedioxy)-19-estra-5(10),9(11)-
diene-17-one (129, multiple vendors) with tBuOK and acetylene
in THF at 0??C gave alcohol 130 in 95% yield, which was subsequently
treated with phenylsulfenyl chloride in the presence of
TEA and AcOH in DCM/CHCl3 at �5??C to 0??C to effect thiolester
formation followed by sulphinate-sulphoxide rearrangement to
give allene sulphoxide 131 in 88% yield. Compound 131 was treated
with NaOMe/MeOH at 64??C to give the corresponding enol
ether and then the enol ether was treated with trimethyl phosphite
at the same temperature for sulphoxide-sulphinate rearrangement
to furnish hydroxyl enol ether 132 in 67% yield. Compound 132
was demethylated with 1 M HCl in methanol to give the corresponding
ketone 133 in 95% yield which was protected using ethylene
glycol, pTsOH and trimethyl orthophosphate in DCM to
afford cyclopentyl ketal 134 in 87% yield. Epoxidation of 134 in
the presence of hexachloroacetone and H2O2 in pyridine and
DCM at 0??C provided a 55:45 mixture of the 5-a,10-a epoxides
(135, 136). The crude epoxides 135 and 136 were reacted with
4-( N,N-dimethylamino)-phenyl magnesium bromide in THF in
the presence of CuCl in DCM to furnish the mixture of diastereomers
137 and 138 in 46% yield over two steps. The mixture (137
and 138) was then treated with KHSO4 in water at 5??C to affect
dehydration and liberation of the keto functional group to give
139 which was used in the next step without isolation. Compound
139 was acetylated with acetic anhydride and perchloric acid in
DCM at �30??C to afford the ulipristal acetate (XXI) in 66% yield
over the final two steps.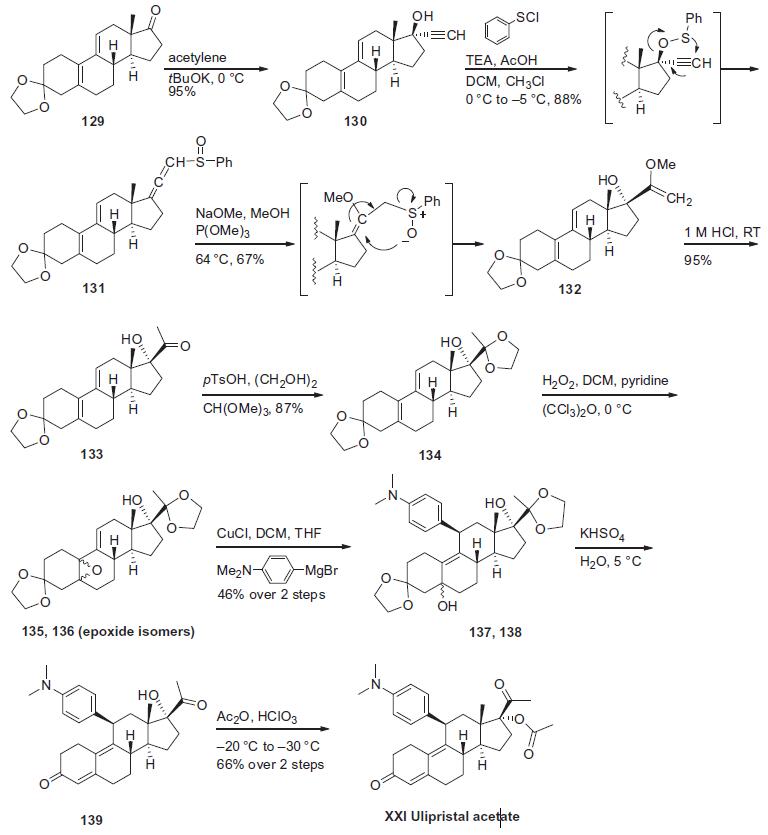
| [storage]
Store at -20°C |
| Questions And Answer | Back Directory | [Description]
Ulipristal acetate (UPA) is a selective progesterone receptor modulator. Attributing to its higher efficacy and a similar rate of side effects compared to levonorgestrel emergency contraception, it is now recommended as first line treatment for emergency contraception. Emergency contraception is defined as the use of drug or device after unprotected or under-protected intercourse to prevent an unwanted pregnancy. Ulipristal acetate can be used up to 5 days (120 h) after unprotected sexual intercourse.
As progesterone promotes the growth of uterine fibroids, the blocking characteristic of ulipristal acetate can thereby be used to reduce the size of uterine fibroids. Ulipristal acetate has also shown efficacy with a significant reduction in uterine bleeding caused by uterine fibroids. The treatment of fibroids by ulipristal acetate should begin in the first week of a menstrual period.
Drugs or herbal products that induce enzymes, including CYP3A4, such as carbamazepine, phenytoin, rifampin, St. John's Wort, etc., may decrease the plasma concentrations of ulipristal acetate, and may decrease its effectiveness while CYP3A4 inhibitors such as itraconazole, ketoconazole, etc., may increase plasma concentrations of ulipristal acetate.
The half-life after oral intake is 32 h; it binds to plasma proteins for 97–99%, and it is metabolized by the citochrome P450.
| [Oral emergency contraception]
Ulinastal acetate is a new oral contraceptive pill, and it is the active chemical composition of Ella, a new generation of emergency contraceptive wildly on the market in United States currently. It can protect women within 120 hours after intercourse without medication, and the emergency contraceptive efficacy will not decline with the delay of treatment time. At the same time, it is safe and durable. Compared with the most commonly used emergency contraceptive levonorgestrel, Ulinastal acetate is more suitable for clinical use, and has the potential to prevent more unwanted pregnancies.
Ulipristal acetate was developed by HRA Pharmaceuticals, Inc., and was marketed by the Food and Drug Administration (FDA) in August 2010 under the trade name Ella. It could be used for the protection of unprotected sex or for the pregnancy prevention in the known or suspected contraceptive failure situation within 120h. Ulipristal acetate is a selective progesterone receptor modulator; and mainly it can take effect by inhibiting the ovulation and plays an emergency contraceptive effect. The study found that Ulastatal acetate was more effective than levonorgestrel in suppressing vomiting after dosing before the estimated ovulation day. And it consequently suggests that Ulatastatin acetate might have a stronger emergency contraceptive effectiveness.
Figure 1 for the structure of ulcaritin acetate | [Pharmacological action]
Ulinastal acetate is a progesterone agonist/antagonist that inhibits or delays the ovulation; however, changes in the endometrium may also be a cause of efficacy.
Ulinastal acetate belongs to the category of selective progesterone receptor modulator, and it has partial agonistic action to progesterone receptor antagonism and partial agonism. It binds to progesterone receptors in the human body and prevents progesterone receptor binding. The efficacy of Ulinastal acetate depends on the time of administration in the menstrual cycle. Administration during the mid-follicular hyperplasia would suppress follicle formation and decrease the estradiol concentrations. And the administration during the in the peak period of follicular hyperplasia can delay the breakdown of follicles from 5 to 9 days. Although early luteal phase of administration cannot significantly delay the endometrial maturation, it can reduce the thickness of the endometrium within 0.6±2.2mm(average value ±SD). | [Pharmacokinetics]
Absorption: The highest fasting plasma concentrations of 20 women reached 176 ng/ mL and 69ng/ mL after 0.9 and 1 hour of using ulipristal acetate and active metabolite (mono-demethyl-ulipristal acetate) in single bolus medication. And mono palmitoyl-ulipristal acetate was lower by 40% to 45% compared with the high-fat breakfast. And ompared with the fasting state, and the delayed tmax (delayed from median 0.75 h to 3 h) with an average AUC0-∞ of 20% to 25% higher. These differences are not expected to impair the efficacy or safety of Ulinastal acetate. Therefore, Ulatastatin acetate can take effect in both situation: with or without food.
Distribution: This product has a high binding rate (> 94%) with plasma protein, high-density lipoprotein, α-1-acid glycoprotein and albumin.
Metabolism: Ulipristal acetate could be metabolized to mono-desmethyl and bis-nor-metabolites. In vitro data indicates that single demethyl metabolites are pharmacologically active, mainly due to CYP3A4 mediated.
Excretion: The terminal half-life of ulipristal acetate in plasma after a single dose of Ulistatta 30 mg would be estimated to (32.4 ± 6.3) hours.
The pharmacological effects, pharmacokinetics, market prospect and patent intellectual property rights of oral emergency contraceptive Ulinastal acetate were edited by Xiao Nan from Chemical book. | [market prospect]
Emergency contraception, also known as after-birth control pills, is a kind of contraceptive for women of childbearing age in the situation od non-protective intercourse or contraceptive failure after the use of contraception to prevent accidental pregnancy.
At present, the most widely used emergency contraceptive is levonorgestrel (1evonorgestre1), which is used at intervals of 12hours each serving one piece (0.75mg) or a single taking of two pieces(1.5mg). However, levonorgestrel was only approved for using as an emergency contraceptive only 72 hours (3days) after unprotected intercourse or contraceptive failure. Even though, the efficacy of levonorgestrel emergency contraception will be significantly reduced with the delay of treatment time; it is recommended that it should better used in unprotected intercourse or contraceptive failure within 12h after the start of medication.
In May 2009, the European Union approved a new emergency contraceptive ulipristal acetate, also called as Ella One, which is not only allowed to be taken within 120 hours (5 days) of unprotected intercourse or contraceptive failure, but the emergency contraceptive efficacy would not delay and decline with the medication time.
Ulastat is one of the first oral contraceptives approved worldwide to be administered within 120 hours of unprotected intercourse or contraceptive failure, which has the potential to prevent more unwanted pregnancies than levonorgestrel. As a new oral emergency contraceptive, Ulinastal acetate can not only be used within 120 hours after unprotected intercourse, but the efficacy of emergency contraception would not decrease with the delay of administration, and it is safe and durable as well. Compared with the most commonly used emergency contraceptive levonorgestrel, Ulinastal acetate is more suitable for clinical use, and it has the potential to prevent more unwanted pregnancies. | [intellectual property rights]
The original research manufacturer in China has already applied for a patent for Ulinastal acetate tablets and its indications as well as for its indications. However, it has not been authorized yet. There are not any intellectual property barriers. | [Other clinical researches]
February 2, 2012, The New England Journal of Medicine published two clinical trials found that Ulinastal acetate has an excellent effect in the treatment of uterine fibroids. The results show that Ulinastal acetate is as effective as leuprolide in controlling uterine fibroid bleeding, reducing the volume of myoma and comforting the pain;
According to statistics, about a quarter of women have suffered from uterine fibroids symptoms. Leuprolide acetate, a gonadotropin-releasing hormone (GnRH) agonist, has been approved for preoperative treatment of uterine fibroids since its approval in 1995. Ulinastal acetate can successfully challenge leuprolide and it becomes become a new choice for preoperative uterine fibroids drug therapy. Let’s wait for the post-trial to reveal the answer. | [References]
[1] Shilpa P. Jadav, Dinesh M. Parmar (2012) Ulipristal acetate, a progesterone receptor modulator for emergency contraception, J Pharmacol Pharmacother, 3, 109-111
[2] Ulipristal acetate for fibroids, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5155051/
[3] Elena Rosato, Manuela Farris, Carbo Bastianeli (2015) Mechanism of Action of Ulipristal Acetate for Emergency Contraception: A Systematic Review, Front Pharmacol, 6, 315
[4] https://www.glowm.com/Critical_current_issue/page/5
|
|
|