| Identification | More | [Name]
Pregabalin | [CAS]
148553-50-8 | [Synonyms]
(3S)-3-(AMINOMETHYL)-5-METHYLHEXANOIC ACID
3(S)-(AMINOMETHYL)-5-METHYLHEXANOIC ACID
PREGABALIN
Pregablin
3-(Aminomethyl)-5-methyl-hexanoic acid
PREDNISOLONESODIUMPHOSPHATE
(R)-Pregabalin
(S)-Pregabalin
(S)-3-(Ammoniomethyl)-5-methylhexanoate
Lyrica | [EINECS(EC#)]
604-639-1 | [Molecular Formula]
C8H17NO2 | [MDL Number]
MFCD00917044 | [Molecular Weight]
159.23 | [MOL File]
148553-50-8.mol |
| Chemical Properties | Back Directory | [Appearance]
Off-White Solid | [Melting point ]
194-196°C | [alpha ]
D23 +10.52° (c = 1.06 in water) | [Boiling point ]
274.0±23.0 °C(Predicted) | [density ]
0.997±0.06 g/cm3(Predicted) | [Fp ]
9℃ | [storage temp. ]
2-8°C | [solubility ]
deionized water: ≥10mg/mL | [form ]
white powder | [pka]
4.23±0.10(Predicted) | [Water Solubility ]
Soluble to 100 mM in water | [Usage]
S-Enantiomer of Pregabalin. A GABA analogue used as an anticonvulsant | [BCS Class]
1 | [InChI]
InChI=1S/C8H17NO2/c1-6(2)3-7(5-9)4-8(10)11/h6-7H,3-5,9H2,1-2H3,(H,10,11)/t7-/m0/s1 | [InChIKey]
AYXYPKUFHZROOJ-ZETCQYMHSA-N | [SMILES]
C(O)(=O)C[C@@H](CN)CC(C)C | [CAS DataBase Reference]
148553-50-8(CAS DataBase Reference) |
| Questions And Answer | Back Directory | [Description]
Pregabalin is a second- generation antiepileptic drug (AED) known with the proprietary brand name of Lyrica® (Pfizer, Tadworth) in the UK and USA (Pfizer, New York, NY).
| [Generic formulation]
MHRA/ CHM advice to minimize risk when switching patients with epilepsy between different manufacturers’ products (including generic products):
- It is usually unnecessary to ensure that patients are maintained on a specific manufacturer’s product unless there are specific concerns, such as patient anxiety and risk of confusion/ dosing error.
| [Indications]
Epilepsy
Adjunctive therapy of focal seizures with and without secondary generalization.
Recommendations summarized from NICE (2012)
- Seizure types—on referral to tertiary care (focal seizures), contraindicated (generalized tonic- clonic seizures, tonic/ atonic seizures, absence seizures, myoclonic seizures).
- Epilepsy types—on referral to tertiary care (benign epilepsy with centrotemporal spikes, panayiotopoulos syndrome, late- onset childhood occipital epilepsy), contraindicated (absence syndromes, idiopathic generalized epilepsy, juvenile myoclonic epilepsy, Dravet syndrome, Lennox– Gastaut syndrome).
Psychiatry
Generalized anxiety disorder.
Neurology
Peripheral and central neuropathic pain.
| [Dose titration]
- Epilepsy— adjunctive therapy: 25 mg bd for 7 days, to be increased by 50 mg every 7days; usual maintenance 300 mg daily, divided into 2 or 3 doses (max. 600 mg daily, divided into 2 or 3 doses).
- Generalized anxiety disorder: 150 mg daily, divided into 2 or 3 doses, for 7 days, to be increased by 150 mg every 7 days (max. 600 mg daily, divided into 2 or 3 doses).
If stopping pregabalin, it is recommended to taper over at least 1 week to avoid abrupt withdrawal.
| [Plasma levels monitoring]
Pregabalin pharmacokinetics are linear over the recommended daily dose range; inter- subject pharmacokinetic variability for pregabalin is low (<20%) and multiple dose pharmacokinetics are predictable from single- dose data. Therefore, there is no need for routine monitoring of plasma concentrations of pregabalin.
| [Antiepileptic drus and therapeutic drugs for neuropathic pain]
Pregabalin is a new antiepileptic drug, having a γ-amino butyric acid structure on its molecular structure, which has anticonvulsant effects, and is successfully developed by the company Pfizer for the treatment of peripheral neuropathic pain, or adjuvant treatment of partial seizures.
In December 2008, the US Food and Drug Administration (FDA) approved pregabalin (trade name "Lyrica") for the treatment of diabetic peripheral neuropathic pain (DPN) and postherpetic neuralgia (PHN)which are Both the most common neuropathic pains.
Neuropathic pain is one of the most difficult chronic pain syndromes to treat , dull pain, burning, tingling as the main feature, there are a lot of incentives of neuralgia, diabetes, infections (such as herpes zoster), cancer and AIDS, etc. can cause neurological pain, in Europe about 3% of the population suffer from neuralgia torture.
The above information is edited by the chemicalbook of Tian Ye.
| [Cautions]
- Patients with conditions that may precipitate encephalopathy.
- Patients with severe congestive heart failure.
| [Adverse effects]
Pregabalin can be associated with adverse effects at the level of the nervous system and other systems.
| [Interactions]
- Since pregabalin is predominantly excreted unchanged in the urine, undergoes negligible metabolism in humans, does not inhibit drug metabolism in vitro, and is not bound to plasma proteins, it is unlikely to produce or be subject to pharmacokinetic interactions.
- Pregabalin may potentiate the effects of lorazepam.
- In the post- marketing experience, there are reports of respiratory failure and coma in patients taking pregabalin and other central nervous system depressant medicinal products. Pregabalin appears to be additive in the impairment of cognitive and gross motor function caused by oxycodone.
With alcohol/food
- There are no specific foods that must be excluded from diet when taking pregabalin.
- Pregabalin may potentiate the effects of alcohol.
| [Special populations]
Hepatic impairment
No dose adjustment is required for patients with hepatic impairment.
Renal impairment
Reduce maintenance dose according to degree of reduction in creatinine clearance.
Pregnancy
-
There is no adequate data from the use of pregabalin in pregnant women. The potential risk for reproductive toxicity in humans is unknown. Pregabalin should not be used during pregnancy unless the benefit to the mother clearly outweighs the potential risk to the foetus.
-
Pregabalin is excreted into human milk. The effect of pregabalin on newborns/ infants is unknown. A case- by- case decision must be made whether to discontinue breast- feeding or to discontinue pregabalin therapy taking into account the benefit of breastfeeding for the child and the benefit of therapy for the woman.
| [Behavioural and cognitive effects in patients with epilepsy]
Pregalin is characterized by a good behavioural profile. This AED does not appear to have significant negative effects on mood or behaviour in patients with epilepsy, although depression has been reported in some patients (dose- dependent effects of mild- to- moderate intensity). A potential abuse or misuse of pregabalin has also been reported, with implications in terms of dependence and withdrawal. pregabalin is also associated with limited negative cognitive effects, mainly related to sedation, decreased arousal, decreased attention and concentration (dose- dependent effects of mild- to- moderate intensity).
| [Psychiatric use]
Pregabalin has an approved indication and is widely used for the treatment of generalized anxiety disorder. Several randomized, double- blind, placebocontrolled trials found that pregabalin is an effective treatment for patients with generalized anxiety disorder and social anxiety disorder. Possible implications in the treatment of mood disorders and benzodiazepines dependence are emerging. Moreover, pregabalin may be a therapeutic agent for the treatment of alcohol abuse, in both withdrawal phase and relapse prevention.
|
| Hazard Information | Back Directory | [Chemical Properties]
Off-White Solid | [Originator]
Warner-Lambert (US) | [Uses]
Pregabalin is an anticonvulsant drug used for neuropathic pain, as an adjunct therapy for partial seizures, and in generalized anxiety disorder. It was designed as a more potent successor to gabapentin. Pregabalin is marketed by Pfizer under the trade nam | [Uses]
S-Enantiomer of Pregabalin. A GABA analogue used as an anticonvulsant. Anxiolytic analgesic used to treat peripheral neuropathic pain and fibromyalgia. | [Definition]
ChEBI: Pregabalin is a gamma-amino acid that is gamma-aminobutyric acid (GABA) carrying an isobutyl substitutent at the beta-position (the S-enantiomer). Binds with high affinity to the alpha2-delta site (an auxiliary subunit of voltage-gated calcium channels) in central nervous system tissues. It has a role as an anticonvulsant and a calcium channel blocker. It is functionally related to a gamma-aminobutyric acid. | [Brand name]
Lyrica (CP). | [General Description]
Pregabalin, marketed as the anticonvulsant drug Lyrica, is used in the treatment of epilepsy and generalized anxiety disorder. Along with other anticonvulsants such as gabapentin and levetiracetam, pregabalin is also used to treat neuropathic pain. This certified reference standard is suitable for pregabalin GC/MS or LC/MS applications from forensic analysis, clinical toxicology and pain prescription monitoring to urine drug testing. | [Biochem/physiol Actions]
Pregabalin is a lipophilic GABA analog/ligand at α2δ subunit of voltage-dependent Ca2+ channels. Pregabalin is an anticonvulsant, anxiolytic analgesic used to treat peripheral neuropathic pain and fibromyalgia. | [Clinical Use]
Antiepileptic
Neuropathic pain
Generalised anxiety disorder | [Synthesis]
Several syntheses of pregabalin (X) have been disclosed in the literature, including process scale-up comparison of
several different routes. The most cost efficient route
as described in the publication is shown in the Scheme.
Condensation of diethyl malonate 69 in the presense of
diisopropyl amine in acetic acid gave a,b-unsaturated diester
70 in high yield. Reaction of the enone diester with
potassium cyanide gave cyano diester 71 in 95% yield. In a
remarkable three step, one pot process, the nitrile in 71 was
hydrolyzed followed by decarboxylation of one of the esters
to provide 72 in 73% yield. Resolution of the two
enantiomers was achieved using (S)-(+)-mandellic acid, one
of the best acid found after many salt screening, to give, after
two recrystallization, a 99:1 ratio of the desired diastereomer.Removal of the acid was done with wet THF instead of base
separation, to avoid salt impurities, and one recrystallization
in ethanol gave 100% ee diastereomer in 25 ¨C 29% overall
yield.
It?ˉs worth noting that the Pfizer group have come up
with a new process of preparing pregabalin (X) via
enantioselective reduction, that promises to further reduce
cost and waste associated with the manufacture of this drug.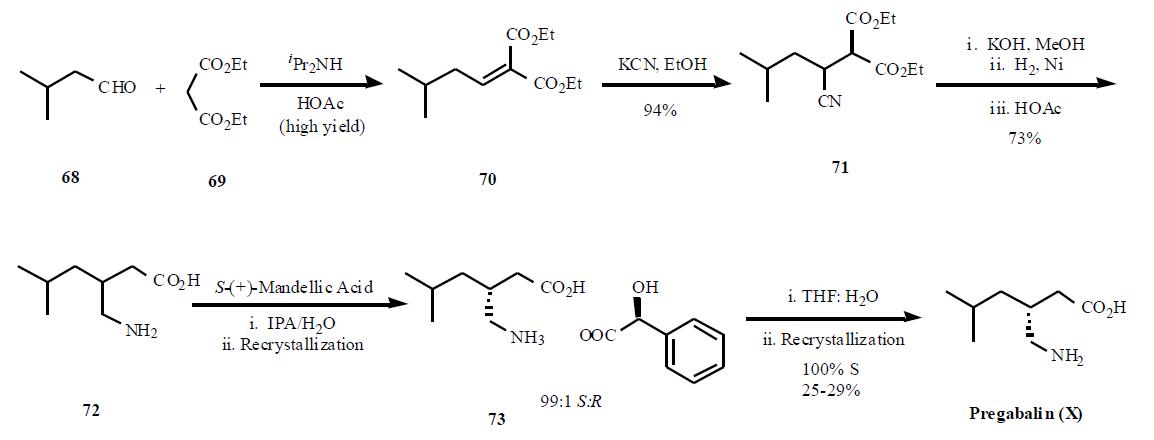
| [target]
Estrogen receptor | Calcium Channel | GABA Receptor | Antifection | 5-HT Receptor | Progestogen receptor | [Drug interactions]
Potentially hazardous interactions with other drugs
Antidepressants: anticonvulsant effect antagonised.
Antimalarials: anticonvulsant effect antagonised by
mefloquine.
Antipsychotics: anticonvulsant effect antagonised.
Orlistat: possible increased risk of convulsions. | [Metabolism]
Pregabalin undergoes negligible metabolism, and about
98% of a dose is excreted in the urine as unchanged drug. |
|
|