| Identification | More | [Name]
Fulvic acid | [CAS]
479-66-3 | [Synonyms]
4,10-dihydro-3,7,8-trihydroxy-3-methyl-10-oxo-1h,3h-pyrano[4,3-b][1]benzopyran-9-carboxylic acid
Fulvic acid
FULVIC ACID PURITY 95%
1H,3H-Pyrano4,3-b1benzopyran-9-carboxylic acid, 4,10-dihydro-3,7,8-trihydroxy-3-methyl-10-oxo- | [EINECS(EC#)]
610-395-7 | [Molecular Formula]
C14H12O8 | [MDL Number]
MFCD09838488 | [Molecular Weight]
308.24 | [MOL File]
479-66-3.mol |
| Questions And Answer | Back Directory | [Overview]
Fulvic acids refer to collectively of a set of organic acids, natural compounds, and components of the humus [which is a fraction of soil organic matter].[1] They share similar structure with humic acids, with differences being the carbon and oxygen contents, acidity, and degree of polymerization, molecular weight, and color.[2] Fulvic acid remains in solution after removal of humic acid from humin by acidification[3, 4]. Humic and fulvic acids are mainly produced by biodegradation of lignin containing plant organic matter. They are not single acids but rather a complex mixture of many different acids containing functional groups that will react as a dibasic acid [2 replaceable hydrogen atoms like H2SO4] or as a tribasic acid [3 replaceable hydrogen atoms like H3PO4].[5]
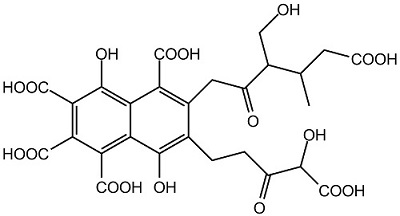
Figure 1 the chemical structure of Fulvic acid; | [Sources]
Fulvic acid can reach high concentrations in solution in poorly drained areas such as bogs and swamps[6] and can sometimes be seen in streams where the water colour would be brown but clear. Humus from geological deposits such as black coal when oxidized, brown coal or lignite is composed of high humic acid but low fulvic acid contents due to leaching. Due to the solubility of fulvic acids in water and the fact that it easily leaches out of source material, it is usually only present in very low concentrations[0.2 – 1% w/v] in leonardite, peat, compost etc. sources. Some companies will dry fulvic acids to a powder, but drying is usually a costly practice that will reflect in the price of these products. Currently in South Africa, a high concentration fulvic acid product, from a sustainable renewable resource has shown to have high ion charge neutralizing and biological activities[6].
| [Humic and Fulvic acid]
Humic Acid and Fulvic Acid represent organic compounds having bio-stimulant role. They are both used to improve nutrient absorption and they are largely used in the supplement industry in different forms. They attach to organic molecules or mineral ions and they hold firmly not to be dissolved in soil but loosely for plants to use them as needed[7].
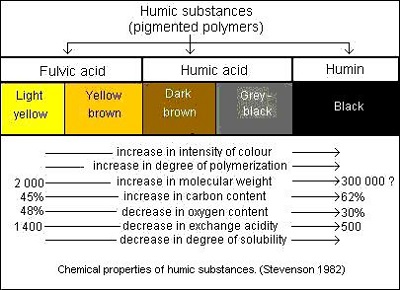
Figure 2 chemical properties of Fulvic acid and humic acid[8].
Humic acids are considered to be the high molecular weight, less oxidized black to dark brown substances and which is the fraction that is not soluble in water under acidic conditions[pH < 2] but is soluble at higher pH values. Humic acids are the major extractable component of soil HS and are the primary organic compounds of soil[humus, peat, compost, manure and coal][9] Commercial humic acid products are usually a salt of Potassium, Sodium or Ammonium and due to the fact that it is extracted with the hydroxides of these ions, the solutions are alkaline and at a pH above 10. In productive agricultural soils, where the pH ranges between 5 and 7, a large portion of the humic acids from these sources occurs as precipitated particles that cannot be taken up by plant roots. It can then only assist in enhancing the uptake and utilization of nutrient minerals from the soil by binding the charged minerals on its ion exchange sites and prevent it from reacting with phosphate anions[Ca2+, Mg2+, Fe2+, Cu2+, Zn2+] to form insoluble phosphate compounds or with sulphate anions to form poorly soluble gypsum[CaSO4]2[10]. It does however contribute towards cation exchange capacity[CEC] in the soil at sufficient concentrations.
Fulvic acids are soluble in water under all pH conditions and remain in solution after removal of humic acid by acidification. Fulvic acids can also be described as being “humic acids” of lower molecular weight and higher oxygen content. The colour of fulvic acids can vary from light yellow to brown in colour. In contrast with humic acids, fulvic acids that are always in solution, especially at the pH of productive agricultural soils, also contribute towards CEC of the soil with the main difference that mineral nutrient fulvate complexes are then in solution and both organic molecule and nutrient ion can be taken up by plant roots and therefore utilized by the plant. Water-soluble nutrients and organic molecules do leach in soils but the rate of leaching as an organic-mineral complex, in relative terms, are much slower compared to the minerals alone in a water solution. Fulvic acids are poly-electrolytes and are unique colloids that diffuse easily through membranes and are therefore easily taken up by plant roots and leaves, whereas all other colloids like humic acid colloids do not.[10] Thus, fulvic acids in comparison to humic acids, contribute directly towards efficient nutrient uptake and utilization from soils. | [Physiochemical property]
The presence of functional groups like carboxylic and phenol groups allows fulvic acids to form complexes with ions such as Mg2+, Ca2+, Fe2+ and Fe3+. Usually fulvic acids have two or more of these groups arranged as to enable the formation of chelate complexes[11, 12]. Elemental characterization of humic fractions on an atomic basis by Helal[2007][13] shows that fulvic acids contains 22% more hydrogen to carbon atoms, 21% more oxygen than carbon and 14% more carboxylic acid groups than humic acids. This implies that fulvic acids are more reactive towards reacting with cations or in other words fulvic acids contain more functional groups of an acidic nature, particularly COOH. The total acidity of fulvic acids[900 1400 meq/100g] are considerably higher than for humic acids[400 870 meq/100g][Yamauchi et al, 1984][10].
| [Applications]
Plant growth stimulation
Fulvic acids as plant biostimulants are mainly produced by biodegradation of lignin containing plant organic matter[14]. Fulvic acids that are always in solution, especially at the pH of productive agricultural soils, also contribute towards cation exchange capacity of the soil[14, 15]. Due to the solubility of fulvic acids in water and the fact that it can be easily leached out, it is usually only present in very low concentrations[0.2-1% w/v] in leonardite, peat, and compost etc, sources. Therefore some companies will dry fulvic acids to a powder[14]. Fulvic acid as an organic fertilizer, is a non-toxic mineral-chelating additive and water binder that maximizes uptake through leaves and stimulates plant productivity[14]. It attracts water molecules, helping the soil to remain moist and aiding the movement of nutrients into plant roots. Fulvic acid easily binds or chelates minerals such as iron, calcium, copper, zinc and magnesium, as it can deliver these elements to plant directly[15]. Lotfi et al.[2015][16] were studied the physiological responses of Brassica napus subjected to fulvic acid application under water stress and found that application of fulvic acid improved the maximum quantum efficiency of PSII[Fv/Fm] and performance index[PI] of plants under both well-watered and limited-water conditions. In various studies, other effects of fulvic acid application were reported such as: enhanced root activity, increase in ion uptake, high rate of transport of phosphorus to the grains[17], increasing the number and length of root hairs of Arabidopsis plants[18, 19], ameliorative growth of rice and radish and higher plant height[20], limiting the development of some pathogens, e.g. Fusarium spp[21], promote plant growth and increase marketable yield in tomato production[22], improved plant growth and yield quantity and quality as well as controlling powdery and downy mildews of cucumber plants and enhanced the activity of soil microorganism[23], enhanced effectively the physiological activities and yield production of tomato plants, as antitranspirants via conserving soil water and thereby reduce the applied water by 25% of irrigation water[24], improve the white rot disease resistance of grape, the quality of berry fruit and more absorption of calcium by grape[25], enhanced potassium levels in leaves of tobacco[26]. Also Yang et al.[2013][27] have demonstrated that fulvic acid is optimum choice for the improvement of P availability and soil physicochemical conditions. Xudan[1987][17] has demonstrated that spraying with fulvic acid can decrease the water stress or the stress imposed by hot, dry winds during ear development and it can increase the grain yield by 7.3-18.0%[17]. Anjum et al.[2011][28] have reported that fulvic acid increased chlorophyll and water content of leaves. It also increased photosynthesis, reduced stomata opening status and transpirations, thus led to growth stimulation and water-loss reduction[29]. Also they have found that fulvic acid and humic acid have been used to regulate the plant growth under well watered and drought conditions. Furthermore, fulvic acid as metabolic anti-transpirations is an organic acid, nontoxic, and not expensive and did not cause pollution problems as a result of extensive use[30].
Powerful Organic Electrolytes
Fulvic acid is an organic natural electrolyte that can balance and energize biological properties it comes into contact with[31]. An electrolyte is a substance that is soluble in water or other appropriate medium that is capable of conducting electrical current[32]. The power of an electrolyte has been shown in repeated tests on animal cells[giant amoebae], to be able to restore life in what researchers termed “a beautiful demonstration” and “astonishing.” When the electrolyte potential was taken away during the test, the cell ruptured and disintegrated into the surrounding fluid causing death. Upon reintroducing electrical potential the cell reconstructed and became active and healthy[33]! Fulvic acid has proven to be a powerful organic electrolyte, serving to balance cell life. If the individual cell is restored to its normal chemical balance and thereby in turn its electrical potential, we have given life where death and disintegration would normally occur within plant and animal cells[34]. Fulvic acid has the outstanding ability to accomplish this objective in numerous ways[35].
Promotes Electrochemical Balance as Donor or Receptor
Fulvic acid is available at times as an electron donor and at other times as an electron acceptor, based on the cell’s requirements for balance[36]. One of the reactions that occurs is an oxidation reaction in which the chemical species loses electrons as a donor. The other reaction is a reduction in which the active species gains electrons as an acceptor[37]. A recent study of the binding of a donor molecule to Fulvic acid in solution revealed direct evidence for donor-acceptor charge transfer mechanisms[38]. Trace minerals in the Fulvic acid electrolyte could also be beneficial in this process by serving as electrodes[39].
Complexes & Dissolves Minerals & Trace Elements[40]
Fulvic acid is especially active in dissolving minerals and metals when in solution with water. The metallic minerals simply dissolve into ionic form, and disappear into the Fulvic structure becoming bio-chemically reactive and mobile. The Fulvic acid actually transforms these minerals and metals into elaborate Fulvic acid molecular complexes that have vastly different characteristics from their previous metallic mineral form. Fulvic acid is nature’s way of “chelating” metallic minerals, turning them into readily absorbable bio-available forms. Fulvic acid also has the unique ability to weather and dissolve silica that it comes into contact with.
Promote assimilation and metabolism
Fulvic acid metal organic complexes are of a low molecular weight, and because of this they are also of low molecular size, and are capable of a high degree of penetration into cells. Fulvic acid complexes and chelates are able to readily pass through semi permeable membranes such as cell walls. Yet it is important to note that it has also been determined that Fulvic acids not only have the ability to transport nutrients through cell membranes, they also have the ability to sensitize cell membranes and various physiological functions as well. Fulvic acid appears to cause the genetic mechanism of plants to function at a higher level. It has been concluded that any means by which plant cells are exposed to Fulvic acid can improve growth.41 Oxygen is absorbed more intensely in the presence of Fulvic acids.42 Fulvic acid aids in penetrating roots and then quickly transports to the shoots of plants.43 Fulvic acid relieves oxygen deficiency and increases the vital activity of cells. Fulvic acids change the pattern of the metabolism of carbohydrates, resulting in an accumulation of soluble sugars. These soluble sugars increase the pressure of osmosis inside the cell wall and enable plants to withstand wilting. Fulvic acid enhances growth and may stimulate the immune system.44
Detoxifies Pollutants[44, 45]
An important aspect of humic substances is related to their sorptive interaction with environmental chemicals, either before or after they reach concentrations toxic to living organisms. The toxic herbicide known as Paraquat is rapidly detoxified by humic substances[Fulvic acids]. Fulvic acids have a special function with respect to the demise of organic compounds applied to soil as pesticides. It has been established that Fulvic acid is vital in helping to form new species of metal ions, binding with organic pollutants such as pesticides and herbicides, and catalyzing the breakdown of toxic pollutants. Radioactive substances react rapidly with Fulvic acid, and only a brief time is required for equilibrium to be reached. All radioactive elements are capable of reacting with Fulvic acid and thus forming organo metal complexes of different adsorptive stability and solubility.
Transports Nutrients[46, 47]
Fulvic acid readily complexes with minerals and metals making them available to plant roots and easily absorbable through cell walls. It makes minerals such as iron, which are not usually very mobile, easily transported through plant structures. Fulvic acids also dissolve and transport vitamins, coenzymes, auxins, hormones, and natural antibiotics that are generally found throughout the soil, making them available. These substances are effective in stimulating even more vigorous and healthy growth. These substances are produced by certain bacteria, fungi, and actinomycetes in decomposing vegetation in the oil. It has been determined that all known vitamins can be present in healthy soil. Plants manufacture many of their own vitamins, yet these from the soil further supplement the plant. Upon ingestion these nutrients are easily absorbed by animals and humans, due to the fact that they are in the perfect natural plant form as nature intends. Fulvic acid can often transport many times its weight in dissolved minerals and elements.
| [Reference]
- Bremner, J. M.[1951-01-01]. "A Review of Recent Work on Soil Organic Matter Part I". Journal of Soil Science. 2[1]: 67–82. doi:10.1111/j.1365-2389.1951.tb00591.x. ISSN 1365-2389.
- http://karnet.up.wroc.pl/~weber/kwasy2.htm
- Aiken, G. R.; McKnight, D. M.; Thorn, K. A.; Thurman, E. M.[1992-07-01]. "Isolation of hydrophilic organic acids from water using nonionic macroporous resins". Organic Geochemistry. 18[4]: 567–573. doi:10.1016/0146-6380[92]90119-I.
- Chefetz, Benny; Chen, Yona; Hadar, Yitzhak; Hatcher, Patrick G.[1998-03-04]. "Characterization of Dissolved Organic Matter Extracted from Composted Municipal Solid Waste". Soil Science Society of America Journal. 62[2]: 326.
- DROZD J. 1978. ZESZ. NAUK. AR WROC?AW, ROZPR. 13: 64 PP.
- https://books.google.com/books?id=H4PtCAAAQBAJ&pg=PA289&lpg=PA289&dq=Fulvic+acid+bogs+and+swamps&source=bl&ots=A76_eqb0wX&sig=x3OE14vz_mOvpKB4eWGShoN_81o&hl=zh-CN&sa=X&ved=2ahUKEwigmrCej_bdAhUbIIgKHWNFBqIQ6AEwAXoECAgQAQ
- http://www.ahealthyjalapeno.com/humic-acid-vs-fulvic-acid/
- STEVENSON F.J.: HUMUS CHEMISTRY GENESIS, COMPOSITION, REACTIONS. WILLEY INTERSCIENCE, NEW YORK 1982.
- STEVENSON F.J.[1994]. HUMUS CHEMISTRY: GENESIS, COMPOSITION, REACTIONS. NEW YORK: JOHN WILEY & SONS.
- YAMAUCHI, MASASHIGE; KATAYAMA, SADAMU; TODOROKI, TOSHIHARU; WATANABLE, TOSHIO[1984]. "TOTAL SYNTHESIS OF FULVIC ACID". JOURNAL OF THE CHEMICAL SOCIETY, CHEMICAL COMMUNICATIONS[23]: 1565-6.
- STEVENSON FJ. ORGANIC FORMS OF SOIL NITROGEN. IN: WILEY JOHN, EDITOR. HUMIC CHEMISTRY: GENESIS, COMPOSITION, REACTION. NEW YORK: 1994. PP. 59–95.
- VERMEER AWP. INTERACTIONS BETWEEN HUMIC ACID AND HEMATITE AND THEIR EFFECTS ON METAL ION SPECIATION. THE NETHERLANDS: WAGENINGEN UNIVERSITY; 1996.[PHD THESIS]
- A.A. HELAL J. SAUDI CHEM. SOC., 11/3[2007], PP. 377–386
- Malan, C.[2015] Review: humic and fulvic acids. A Practical Approach.In Sustainable soil management symposium. Stellenbosch, 5-6 November 2015, Agrilibrium Publisher.
- Yamauchi, M., Katayama, S., Todoroki, T., Watanable, T.[1984] Total synthesis of fulvic acid. Journal of the Chemical Society, Chemical Communications. 23, 1565-1576.
- Lotfi, R., Pessarakli, M., Gharavi-Kouchebagh, P., Khoshvaghti, H.[2015] Physiological responses of Brassica napus to fulvic acid under water stress: Chlorophyll a fluorescence and antioxidant enzyme activity. The Crop Journal, 3, 434 439.
- Xudan, X.[1987] The effect of foliar application of fulvic acid on water use, nutrient uptake and yield in wheat. Australian Journal of Agricultural Research, 37, 343-350.
- 18. Schmidt, W., Cesco, S., Santi, S., Pinton, R., Varanini, Z.[2005] Water-extractable humic substances as nutrient acquisition signals for root hairs development in Arabidopsis. In: Hartmann, A.,
- Schmid, M., Wenzel, W., Hinnsinger, P.[2004] Rizosphere Perspectives and Challenges, 1171-1178, Elsevier.
- Khang, V.T.[2011] Fulvic foliar fertilizer impact on growth of rice and radish at first stage. Omonrice, 18, 144-148.
- Yigit, F., Dikilita?, M.[2008] Effect of humic acid applications on the root-rot diseases caused by Fusarium spp. on tomato plants. Plant Pathology, 7[2], 179-182.
- Suh, H. Y., Yoo, K. S., Suh, S. G.[2014] Effect of foliar application of fulvic acid on plant growth and fruit quality of tomato[Lycopersicon esculentum L.]. Horticulture, Environment, and Biotechnology, 55[6], 455–461.
- Kamel, S. M., Afifi, M. M. I., El-shoraky, F., El-Sawy, M. M.[2014] Fulvic acid: a tool for controlling powdery and downy mildews in cucumber plants. International Journal of Phytopathology, 3[2], 101-108
- Aggag, A. M., Alzoheiry, A. M., Abdallah, A. E.[2015] Effect of kaolin and fulvic acid antitranspirants on tomato plants grown under different water regimes. Alexandria Science Exchange Journal, 36[2], 169-179.
- 25. Huanpu, M., Baoyan, L., Zhimin, L.[2004] Effects of fulvic acid foliar spray on growth and development of grape. Journal of Beijing Agricultural College, 19[4], 1-3.
- Priya, B. N. V., Mahavishnan, K., Gurumurthy, D. S., Bindumadhavh, A., Ambika, P. U., Navin, K. SH.[2014] Fulvic acid for enhanced nutrient uptake and growth: insights from biochemical and genomic studies. Journal of Crop Improvement, 28,740–757.
- Yang, S., Zhang, Z., Cong, L., Wang, X., Shi, S.[2013] Effect of fulvic acid on the phosphorus availability in acid soil. Journal of Soil Science and Plant Nutrition, 13[3], 526-533.
- Anjum, S. A., Wang, L., Farooq, M., Xue, L., Ali, S.[2011] Fulvic acid application improves the maize performance under well-watered and drought conditions. Journal of Agronomy and Crop Science, 197[6], 409417.
- Li, M.S., Li, S., Zhang, B. L. C.[2005] Physiological effect of new FA antitranspirant on winter wheat at ear filling stage. Journal of Agricultural sciences in China, 11, 820-825.
- Nardi, S., Pizzeghello, D., Muscolo, A., Vianello, A.[2002] Physiological effects of humic substances on higher plants. Soil Biology & Biochemistry, 34, 1527–1536.
- Vital electrolytes – Baker, W.E.[1973]. Geochimica et Cosmochimica Acta, 37, 269-281.
- Gamble, D.S., & Schnitzer, M.[1974]. Trace Metals and Metal-Organic Interactions in Natural Waters. Ann Arbor, Mi: Ann Arbor Science.
- Power of an electorlyte – Crile, G.[1926]. A bipolar theory of living porcesses. New York: McMillan.
- powerful electrolyte – Jackson, William R.[1993]. Humic, Fulvic and Microbial Balance: Organic Soil Conditioning, 329. Evergeen, Colorado: Jackson Research Center.
- New Electronic Encyclopedia.[1991]. Photosynthesis. Grolier Electronic Publishing.
- Donor and receptor – Rashid, M.A.[1985]. Geochemistry of marine humic substances. New York: Springer-Verlag.
- Donor, receptorSposito, G., Holtzclaw, K.M., LeVesque, C.S., & Johnston, C.T.[1982]. Trace metal chemistry in aridzone field soils amended with sewage sludge. II. Comparative study of the fulvic acid fraction. Soil Science Society America Journal, 46. 265-270.
- Mineral complexes in fulvic may serve as electrodes – Rashid, M.A.[1985]. Geochemistry of marine humic substances. New York: SpringerVerlag.
- Free radical – Senesi, N.[1990] Analytica Chmica Acta, 232, 51-75. Amsterdam, The Netherlands: Elsevier.
- Dissolves metals and minerals – Ong, H.L., Swanson, V.D., & Bisque, R.E.[1970] Natural organic acids as agents of chemical weathering[130170]. U.S. Geological Survey Professional Paper 700 c. Washngton, DC: U.S. Geological Survey.
- Genetic and growth-Jackson, William R.[1993]. Humic, Fulvic and Microbial Balance: Organic Soil Conditioning, 538. Evergreen, Colorado: Jackson Research Center.
- Oxygen is absorbed – Kononova, M.M.[1966]. Soil organic matter. Elmsford, NY: Pergamon.
- Rapid transport to shootsKononova, M.M.[1966]. Soil organic matter. Elmsford, NY: Pergamon
- immune system – Syltic, P.W.[1985]. Effects of very small amounts of highly active biological substances on plant growth. Biological Agriculture and Horticulture, 2, 245-269; and, Research reports and studies, Appropriate Technology Ltd. Dallas, TX: Murray Sinks II of ATL[Publisher].
- Modify damage by toxic compounds – Christman, R.F., & Gjessing, E.T.[1983]. Aquatic and terrestrial humic materials. The Butterworth Grove, Kent, England: Ann Arbor Science.
- Prakash, A.[1971]. Terrigenous organic matter and coastal phytoplankton fertility. In J.D. Costlow[Ed.], Fertility of the sea, 2, 351-368.[Proceedings of an International Symposium on Fertility of the Sea, Sao Paulo, Brazil, London, and New York: Gordon and Breach Science]
- Enhance and transport nutrients – Prakish, A.[1971]. Fertility of the Sea, 2, 351-368.
- Williams, S. T.[1963]. Are antibiotics produced in soil? Pedobiologia, 23, 427-435.
|
| Hazard Information | Back Directory | [Uses]
Fulvic acid is an organic and natural electrolyte. It has exhibited the ability to enhance the availability and adsorption of nutrients. | [storage]
+4°C |
|
|