| Identification | More | [Name]
Phenylbutazone | [CAS]
50-33-9 | [Synonyms]
1,2-diphenyl-3,5-dioxo-4-n-butylpyrazoline
4-BUTYL-1,2-DIPHENYL-3,5-PYRAZOLIDINEDIONE
4-BUTYL-1,2-DIPHENYLPYRAZOLIDINE-3,5-DIONE
AURORA KA-3837
BUTADIONUM
PHENYLBUTAZONE
1,2-Diphenyl-2,3-dioxo-4-N-butylpyrazoline
1,2-Diphenyl-3,5-dioxo-4-butylpyrazolidine
1,2-Diphenyl-4-butyl-3,5-dioxopyrazolidine
1,2-Diphenyl-4-butyl-3,5-pyrazolidinedione
3,5-dioxe-4buty-1,diphenyl-pyrazolidine
3,5-dioxo01,2-diphenyl-4-m-butyl-pyrazolidine
3,5-Dioxo-1,2-diphenyl-4-N-butyl-pyrazolidin
3,5-Dioxo-1,2-diphenyl-4-N-butylpyrazolidine
3,5-Pyrazolidinedione, 4-butyl-1,2-diphenyl-
4-Butyl-1,2-diphenyl-3,5-dioxopyrazolidine
4-butyl-1,2-diphenyl-5-pyrazolidinedione
4-n-butyl-1,2-diphenyl-3,5-pyrazolidinedione
A 7514
Alindor | [EINECS(EC#)]
200-029-0 | [Molecular Formula]
C19H20N2O2 | [MDL Number]
MFCD00005500 | [Molecular Weight]
308.37 | [MOL File]
50-33-9.mol |
| Chemical Properties | Back Directory | [Appearance]
Off-Whtie Solid | [Melting point ]
106-108 °C (lit.) | [Boiling point ]
448.76°C (rough estimate) | [density ]
1.1591 (rough estimate) | [refractive index ]
1.6140 (estimate) | [storage temp. ]
2-8°C | [solubility ]
Practically insoluble in water, sparingly soluble in alcohol. It dissolves in alkaline solutions. | [form ]
Powder | [pka]
4.5(at 25℃) | [color ]
White to almost white | [Stability:]
Stable. Incompatible with strong oxidizing agents, strong acids, strong bases. | [Water Solubility ]
<0.1 g/100 mL at 23.5 ºC | [Usage]
A non-steroidal anti-inflammatory compound. An inhibitor of cyclooxygenase that is also a substrate for peroxidation by cyclooxygenase | [Merck ]
14,7277 | [BRN ]
290080 | [InChIKey]
VYMDGNCVAMGZFE-UHFFFAOYSA-N | [LogP]
3.160 | [CAS DataBase Reference]
50-33-9(CAS DataBase Reference) | [IARC]
3 (Vol. 13, Sup 7) 1987 | [NIST Chemistry Reference]
Phenylbutazone(50-33-9) | [EPA Substance Registry System]
50-33-9(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,T | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R42/43:May cause sensitization by inhalation and skin contact .
R45:May cause cancer. | [Safety Statements ]
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S22:Do not breathe dust .
S53:Avoid exposure-obtain special instruction before use . | [RIDADR ]
3249 | [WGK Germany ]
3 | [RTECS ]
UQ8225000 | [TSCA ]
Yes | [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
29331990 | [Safety Profile]
Suspected human
carcinogen producing leukemia. A human
poison by parenteral route. An experimental poison by ingestion, intraperitoneal,
subcutaneous, intravenous, and
intramuscular routes. Human systemic
effects by ingestion and possibly other
routes: fever, blood pressure increase, other
unspecified vascular effects, damage to
kidney tubules and glomeruli, decreased
urine volume, blood in the urine, reduction
in the number of whte blood cells, and
agranulocytosis. Experimental teratogenic
and reproductive effects. Human mutation
data reported. An eye irritant. An antiinflammatory agent. When heated to
decomposition it emits toxic fumes of NOx | [Hazardous Substances Data]
50-33-9(Hazardous Substances Data) | [Toxicity]
LD50 oral in rabbit: 781mg/kg |
| Hazard Information | Back Directory | [General Description]
Odorless white or off-white crystalline powder. Tasteless at first, but slightly bitter aftertaste. pH (aqueous solution) 8.2. | [Reactivity Profile]
PHENYLBUTAZONE(50-33-9) is incompatible with strong oxidizers, strong acids and strong bases. . | [Air & Water Reactions]
This compound is relatively stable at ambient temperatures. Aqueous decomposition of this chemical occurs by hydrolysis and oxidation. Insoluble in water. | [Fire Hazard]
Flash point data for this chemical are not available; however, PHENYLBUTAZONE is probably combustible. | [Description]
Phenylbutazone, one of the earliest NSAIDs
introduced, is now indicated for the symptomatic
relief of rheumatoid arthritis, osteoarthritis, psoriatic arthritis, ankylosing spondylitis, gout, and
acute superficial thrombophlebitis. The gastrointestinal and bone marrow toxicity observed in its early use have been greatly
reduced by lower dosage (300 mg/d). Nevertheless, it is used primarily where other drugs have
failed and then only for short-term therapy. The
drug has a long serum half-life of about 100 h. It
is a moderately active cyclooxygenase inhibitor
and it suppresses both spontaneous and chemotactic motility of neutrophils. In addition to the
serious gastrointestinal and hematological adverse effects, sodium and water retention, rash,
vertigo, and dermatitis are observed. | [Chemical Properties]
Off-Whtie Solid | [Originator]
Butazolidin, Geigy ,US,1952 | [Uses]
A non-steroidal anti-inflammatory compound. An inhibitor of cyclooxygenase that is also a substrate for peroxidation by cyclooxygenase | [Uses]
An inhibitor of Cox. | [Uses]
For the treatment of backache and ankylosing spondylitis | [Uses]
Phenylbutazone, a nonsteroidal anti-inflammatory drug, is an efficient reducing cofactor for the peroxidase activity of COX. Phenylbutazone-dependent inactivation of COX and prostacyclin synthase is markedly increased in the presence of 100 μM hydrogen peroxide with half-maximal effects at Phenylbutazone concentrations of 100 and 25 μM for COX and prostacyclin synthase, respectively. | [Definition]
ChEBI:Phenylbutazone is a member of the class of pyrazolidines that is 1,2-diphenylpyrazolidine-3,5-dione carrying a butyl group at the 4-position. It has a role as a non-narcotic analgesic, a non-steroidal anti-inflammatory drug, an antirheumatic drug, a peripheral nervous system drug, a metabolite and an EC 1.1.1.184 [carbonyl reductase (NADPH)] inhibitor. | [Manufacturing Process]
7.6 parts of sodium are dissolved in 190 parts by volume of absolute alcohol; 65 parts of diethyl-n-butyl malonate and 65 parts of hydrazobenzene are added. The alcohol is slowly distilled off and the reaction mixture heated for 12 hours at a bath temperature of 150°C and finally in vacuo, until no more alcohol comes off.
The product is dissolved in water, clarified with a little animal charcoal and 15% hydrochloric acid is slowly added until an acid reaction to Congo red paper is produced. 1,2-Diphenyl-3,5-dioxo-4-n-butyl-pyrazolidine separates as an oil, which rapidly become crystalline. It crystallizes from alcohol as colorless needles with a MP of 105°C. | [Brand name]
Azolid (Sanofi Aventis); Butazolidin (Novartis);Algesin;Algirreudin;Algoverine;Alka butazolidin;Alkabutazone;Alka-phenylbutazone;Alka-sterazolidin;Anarthral;Apophenylbutazone;Apo-phenylbutazone;Arteopan;Arthirikin;Artibrin;Artrisin;Artrodesmol extra;Bizolin 20;Bizolin 700;Butacal;Butacol;Butadilat;Butadin;Butadyne;Butafenil;Butagros;Butakvertin;Butaparin;Butaphen;Buta-phen;Butarex;Butartiril;Butatril;Butazolidin alka;Butazolidina;Butial;Butinol;Butiwas;Buto beta;Butoroid cream;Butrex;Carudol;Celestalgon;Celestazone;Colfezone;Corbuvit;Dartranol;Debutazon;Delta-butazolidin;Delta-demoplas;Delta-myogit;Delta-tomanol;Deltawaukobuzon;Dephimixn;Dexa tomanol;Dexa-attritin;Dexa-escopyrin;Dexamed;Dexatrzona;Dibuzon;Direstop;Ditrone;Doctofril;Dolosin dexa;Dolpirina;Ectobutazone;Ethibute;Exraheudon;Exrheudon;F 650;Fenibutina;Flebosil;Glycyl;Hepabuzon;Inflazone;Intrabuzone;Mammyl;Megazone;Mepha-butazone;Mepropyrin;Mi 540;Naupax;Neuro-demoplas;Neuzoline m;Novobutazone;Oluprin;Oppazoen;Osadrinim;Parazolidin;Parzolidon;Pasirheuman;Penetradol;Phenbuff;Phenbutazone;Phenylarthrite;Phenylbetazone;Phenylon plus;Phlebolan;Pirabutil;Pirarreumol-b;Pirarreumol-p;Prebutex;Precirhemin;Prednirheumin;Proxyfezone;Proydynam;Pyrbutal;Ranocor;Reopin;Reumilene;Rheopyrin;Rheosolon;Rheumanoln;Rheumaphen;Rheumycalm;Salzone;Servizolidin;Sigma-elmedal;Sintobutina;Stabilat;Tevocodyn;Tibutazone;Ticinil calcio;Ticinil calico;Trabit;Zolapelin;Zolidinium. | [Therapeutic Function]
Antiinflammatory, Antiarthritic | [World Health Organization (WHO)]
Phenylbutazone, a pyrazolone derivative with anti-inflammatory,
analgesic and antipyretic activity, was introduced in 1949 for the treatment of
rheumatic disorders. Its use was subsequently associated with serious and
sometimes fatal adverse reactions, notably cases of aplastic anaemia and
agranulocytosis. Many national drug regulatory authorities consider that more
recently introduced drugs offer a safer alternative for most, if not all, patients
requiring anti-inflammatory agents. Phenylbutazone has thus been either
withdrawn at the national level or retained with rigorously restricted indications for
patients unresponsive to other therapy. These restrictions also apply, in general, to
combination products containing phenylbutazone. | [Biochem/physiol Actions]
Phenylbutazone is a hepatotoxin and binds to plasma proteins. It is used to treat inflammation in horse. Phenylbutazone has potential to treat ankylosing spondylitis. It has therapeutic potential to treat myotonic dystrophy type 1 (DM1) by inducing muscle blind-like protein 1 (MBNL1) expression. It also favors the expression of muscle chloride channel in skeletal muscles. | [Clinical Use]
Phenylbutazone is a
nonsteroidal anti-inflammatory drug used for
the acute treatment of ankylosing spondylitis,
chronic polyarthritis, and gout. Because of
severe side effects including disturbances of
the hematopoietic system like agranulocytosis
and aplastic anemia the use is limited
to conditions in which other nonsteroidal antiinflammatory
drugs do not show sufficient efficacy.
Phenylbutazone is given as oral, rectal,
intramuscular or topical formulation (up to 600
mg/d initial dose, up to 400 mg/d maintenance
dose). The peak plasma concentration is reached
2 h after oral application. Phenylbutazone is
bound to 98% to plasma protein. Oxyphenbutazone
is formed as an active metabolite of phenylbutazone. | [Synthesis]
Phenylbutazone, 4-butyl-1,2-diphenyl-3,5-pyrazolidinedione (3.2.6),
is synthesized in a single stage by reacting hydrazobenzol with butylmalonic ester
[68,69].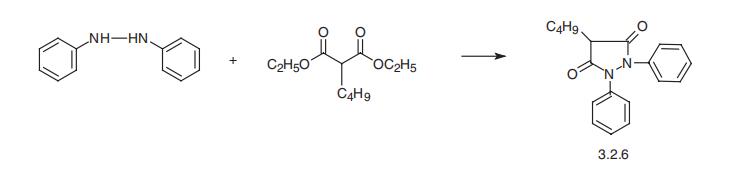
| [Veterinary Drugs and Treatments]
One manufacturer lists the following as the indications for phenylbutazone:
“For the relief of inflammatory conditions associated
with the musculoskeletal system in dogs and horses.” (Package
Insert; Butazolidin?—Coopers). It has been used primarily for the
treatment of lameness in horses and, occasionally, as an analgesic/
antiinflammatory, antipyretic in dogs, cattle, and swine. | [storage]
Store at -20°C | [Purification Methods]
Crystallise the dione from EtOH. Its pK2 3 is 4.52 (in H2O), 4.89 (in 50% aqueous EtOH) and 5.25 (80% 2-methoxyethanol). It complexes with Hg2+, Cd2+ and Zn2+. It has UV with max at 239.5nm in MeOH+50% aqueous HClO4 and 264nm in aqueous 0.1N NaOH. [Beilstein 24 III/IV 1123.] |
| Questions And Answer | Back Directory | [Abstract]
Phenylbutazone, often referred to as "bute,"is a nonsteroidal anti-inflammatory, antipyretic, and analgesic drug (NSAID) for the short-term treatment of pain and fever results from rheumatoid arthritis, ankylosing spondylitis, gouty arthritis, and osteoarthritis.
Phenylbutazone has been removed from the United States market due to the availability of newer drugs with less adverse effects. Phenylbutazone is a nonsteroidal anti-inflammatory drug (NSAID) effective in treating fever, pain, and inflammation in the body. As a group, NSAIDs are non-narcotic relievers of mild to moderate pain of many causes, including injury, menstrual cramps, arthritis and other musculoskeletal conditions. | [Chemical Property]
It is soluble in acetone, chloroform or benzene freely; soluble in ethanol or ether; hardly soluble in water; soluble in sodium hydroxide solution. | [Mechanism of action]
Recent studies have found that the generating mechanism from phenylbutazone anti-inflammatory, analgesic and anti-rheumatic effect is not due to pituitary-adrenal excitement may be due to drugs that inhibit the generation of inflammatory tissue inflammation related active substances, such as synthesis of prostaglandins, white blood cells activities and transfer, release and activity of lysosomal enzymes; pain may also inhibit prostaglandin synthesis results. | [Pharmacokinetic Study]
Oral absorption effect is quickly and completely, reached the peak plasma concentration after about 2 hours. Plasma protein binding rate is 98%. The apparent volume of distribution is 0.12L/kg, such as increasing the dose, volume of distribution has also increased, but the blood concentration does not increase. Therefore, when using repeatedly, the steady-state plasma concentration does not increase linearly. The half-life is 56 to 86 hours. It can cross the placenta into the milk. This product is metabolized by the liver, metabolites is hydroxy phenylbutazone and γ-hydroxy phenylbutazone, still active, The final metabolites are excreted in urine and a small amount of bile is excreted through the urine. | [Attention and Taboo]
The side effects of phenylbutazone incidence rate is about 10%~20%, gastrointestinal irritation can cause nausea, vomiting, abdominal pain, constipation, overdose can cause peptic ulcer, blood in the stool. Damages also occur in other systems, such as rash, dizziness, hematuria, hepatitis, etc. Inhibit the bone marrow caused by neutropenia, or even aplastic anemia, such as the timely withdrawal is more recoverable and therefore should be ground check blood, no effect by 1 week, should not be reused. And double coumarin anticoagulants, sulfonamides, tolbutamide hypoglycemic drugs, increase the plasma concentration, toxicity increases. Sodium, chlorine retention effect, hypertension, edema, heart failure patients cannot use it and limit salt intake during the treatment. Patients with weak liver, osteoporosis, and kidney as well as drug allergy history are contraindicated or appropriate caution. | [Preparation]
Cyclization reaction of hydrogenated azobenzene and diethylmalonic acid diethyl: hydrazobenzene, butyl diethyl malonate, sodium sulfite and methanol are heated with severe reflux 1.5h, born in Bute salt, acidified with acetic acid to give Bute. | [Acute toxicity]
oral-rat LD50: 245 mg/kg; Oral-Mouse LD50: 270 mg/kg
Irritation Data: Eye – rabbit, 100 mg, moderate | [Flammability hazard properties]
thermal decomposition emitted poisonous nitrogen oxides fumes | [Storage characteristics]
Treasury ventilation low-temperature drying; and food raw materials stored separately | [Extinguishing agent]
Water, carbon dioxide, dry powder, foam | [References]
https://en.wikipedia.org/wiki/Phenylbutazone
https://pubchem.ncbi.nlm.nih.gov/compound/phenylbutazone#section=Top
http://www.medicinenet.com/phenylbutazone/article.htm |
|
|