| Identification | More | [Name]
Haloperidol | [CAS]
52-86-8 | [Synonyms]
4-[4-(4-CHLOROPHENYL)-4-HYDROXY-1-PIPERIDINYL]-1-(4-FLUOROPHENYL)-1-BUTANONE
4-[4-(4-CHLOROPHENYL)-4-HYDROXYPIPERIDINO]-4'-FLUOROBUTYROPHENONE
AKOS 91337
HALOPERIDOL
1-(3-p-Fluorobenzoylpropyl)-4-p-chlorophenyl-4-hydroxypiperidine
1-Butanone, 4-[4-(4-chlorophenyl)-4-hydroxy-1-piperidinyl]-1-(4-fluorophenyl)-
1-butanone,4-(4-(4-chlorophenyl)-4-hydroxy-1-piperidinyl)-1-(4-fluorophenyl)
4-(4-(4-chlorophenyl)-4-hydroxy-1-piperidinyl)-1-(4-fluorophenyl)-1-butanon
4-(4-(para-Chlorophenyl)-4-hydroxypiperidino)-4'-fluorobutyrophenone
4-(4-(p-chlorophenyl)-4-hydroxypiperidino)-4’-fluoro-butyrophenon
4-(4-(p-chlorophenyl)-4-hydroxypiperidino)-4’-fluorobutyrophenone
4-(4-(p-Chlorophenyl)-4-hydroxypiperidino)-4'-fluorobutyrophenone
4-(4-hydroxy-4’-chloro-4-phenylpiperidino)-4’-fluorbutyrophenone
4-(4-hydroxy-4’-chloro-4-phenylpiperidino)-4’-fluorobutyrophenone
4-(4-Hydroxy-4'-chloro-4-phenylpiperidino)-4'-fluorobutyrophenone
4-[4-(4-Chlorophenyl)-4-hydroxy-1-piperidinyl]-4’-(4-flurophenyl)-1-butanone
4’-fluoro-4-(4-(p-chlorophenyl)-4-hydroxypiperidino)-butyrophenon
4’-fluoro-4-(4-(p-chlorophenyl)-4-hydroxypiperidinyl)butyrophenone
4’-fluoro-4-(4-hydroxy-4-(4’-chlorophenyl)piperidino)butyrophenone
4’-fluoro-4-(4-hydroxy-4-p-chlorophenylpiperidino)butyrophenone | [EINECS(EC#)]
200-155-6 | [Molecular Formula]
C21H23ClFNO2 | [MDL Number]
MFCD00051423 | [Molecular Weight]
375.86 | [MOL File]
52-86-8.mol |
| Chemical Properties | Back Directory | [Appearance]
White Crystalline Powder | [Melting point ]
152 °C | [Boiling point ]
529.0±50.0 °C(Predicted) | [density ]
1.1820 (estimate) | [Fp ]
9℃ | [storage temp. ]
2-8°C | [solubility ]
45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 0.39 mg/mL
| [form ]
powder
| [pka]
8.3(at 25℃) | [color ]
white
| [Water Solubility ]
2.058mg/L(22.5 ºC) | [Usage]
Antidyskinetic; antipsychotic | [Merck ]
4598 | [BCS Class]
4/3 | [CAS DataBase Reference]
52-86-8(CAS DataBase Reference) | [NIST Chemistry Reference]
Haloperidol(52-86-8) | [EPA Substance Registry System]
52-86-8(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
R60:May impair fertility.
R61:May cause harm to the unborn child.
R25:Toxic if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin .
R43:May cause sensitization by skin contact. | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 2811 6.1/PG 3
| [WGK Germany ]
3
| [RTECS ]
EU1575000
| [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
2933399090 | [Hazardous Substances Data]
52-86-8(Hazardous Substances Data) | [Toxicity]
LD50 orally in rats: 165 mg/kg (Goldenthal); i.p. in mice: 60 mg/kg (Collins, Horlington) |
| Hazard Information | Back Directory | [Description]
Haloperidol is a butyrophenone with a long duration of action. It has lile α-
adrenoceptor blocking activity and minimal effect on the cardiovascular
system. It is an effective antiemetic but has a high incidence of
extrapyramidal adverse effects. Haloperidol may be used in the short-term
management of the acutely agitated patient (when sinister causes of
confusion such as hypoxaemia and sepsis have been excluded) and in the
management of delirium in ICU. The duration of action of
haloperidol is approximately 24–48h. | [Chemical Properties]
White Crystalline Powder | [Originator]
Haldol,Janssen-Le Brun,France,1960 | [Uses]
Antidyskinetic; antipsychotic | [Uses]
Haloperidol is one of the most actively used modern neuroleptics. Its high antipsychotic
activity is combined with a moderate sedative effect. It effectively stops various types of
psychomotor excitement. It is used for schizophrenic psychoses, manic, paranoid, and
delirious conditions, depression, psychomotor excitement of various origins, and for delir�ium and hallucinations of different origin. | [Definition]
ChEBI: A compound composed of a central piperidine structure with hydroxy and p-chlorophenyl substituents at position 4 and an N-linked p-fluorobutyrophenone moiety. | [Manufacturing Process]
A stirred slurry of 120.0 parts 4-(4-chlorophenyl)-piperidin-4-ol hydrochloride
and 40.0 parts of potassium iodide in 500 parts of water is warmed to a
temperature of about 35°C under a nitrogen atmosphere. Then, 70.0 parts of
potassium hydroxide is added. After further heating to about 55°C. 138.0
parts of 1,1 dimethoxy-1-(4-fluorophenyl)-4-chlorobutane is added. The
temperature is then raised to about 102°C and heating continued for 3.5
hours. After cooling to about 75°C. 785 parts of toluene is added to the
reaction mixture and stirred for about 5 minutes. An additional 320 parts of
toluene is added and the water and organic layers separated. 102 parts of
methanol is used to rinse the flask and added to the organic layer to provide a
solution of 4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4,4-dimethoxybutyl]-
piperidin-4-ol. Then, 59 parts of concentrated hydrochloric acid is added to a
stirred solution of the organic layer to precipitate a solid. The solid is filtered,
rinsed twice with 550 parts by volume portions of a 10:9:1 acetone-toluene�methanol mixture, twice with 400 parts by volume portions of a 10:l acetone�methanol mixture, and air-dried. The dried solid is then dissolved in 1,950
parts of methanol with gentle heating on a steam bath. The resulting solution
is filtered and 300 parts by volume of concentrated ammonium hydroxide is
added. Heating is continued to reflux and maintained thereat for about 1 hour.Then, 2,520 parts of water is added and the slurry stirred at about 75°C for
1.5 hours. After cooling to about 25°C. the solid is filtered, washed twice with
600 parts by volume portions of a 3:1 mixture of water-methanol, and air�dried. The resulting product, 4-[4-chlorophenyl)-4-hydroxypiperidino]-4'-
fluorobutyrophenone, is obtained in 32.5% yield. This product melts at about
148.5°C to 150.5°C. | [Brand name]
Haldol (OrthoMcNeil). | [Therapeutic Function]
Antidyskinetic, Antipsychotic | [General Description]
Haloperidol, 4-[4-(p-chlorophenyl)-4-hydroxypiperidino]-4-fluorobutyrophenone (Haldol), is anodorless white to yellow crystalline powder. Haloperidol iswell and rapidly absorbed and has a high bioavailability. It ismore than 90% bound to plasma proteins. Haloperidol is excretedslowly in the urine and feces. About 30% of a dose isexcreted in urine and about 20% of a dose in feces via biliaryelimination,and only 1% of a dose is excreted as unchangeddrug in the urine.Haloperidol is a minor substrate of CYP1A2 and a major substrate of CYP2D6 and CYP3A4.CYP2D6 inhibitors may increase the levels/effects ofhaloperidol.Haloperidol may increase the levels/effects ofCYP2D6 substrates and it may decrease the bioactivationof CYP2D6 prodrugs substrates. Haloperidol also is a moderateinhibitor of CYP2D6 and CYP3A4. CYP3A4 inducersmay decrease the levels/effects of haloperidol, whereasCYP3A4 inhibitors may increase the levels/effects ofhaloperidol. Centrally acting acetylcholinesterase inhibitorsmay increase the risk of antipsychotic-related EPS. The precisemechanism of antipsychotic action is unclear but isconsidered to be associated with the potent DA D2receptor–blocking activity in the mesolimbic system and theresulting adaptive changes in the brain. Haloperidol is usedprimarily for the long-term treatment of psychosis and is especiallyuseful in patients who are noncompliant with theirdrug treatment. | [General Description]
Haloperidol, 4[4-(p-chlorophenyl)-4-hydroxypiperidone]-4' -n-fluorobutyrophenone (Haldol),the representative of several related classes of aromaticbutylpiperidine derivatives, is a potent antipsychotic usefulin schizophrenia and in psychoses associated with braindamage. It is frequently chosen as the agent to terminatemania and often used in therapy for Gilles de la Tourettesyndrome. Haloperidol-induced dyskinesias may involveneurotoxicological metabolite similar to dopaminergic toxicantMPP+. | [Pharmaceutical Applications]
Haloperidol is an analogue of the dopamine D2 receptor antagonist and is an older antipsychotic drug. The drug is used in the treatment of schizophrenia, a neuropsychiatric disorder. In general, antipsychotic drugs work by blocking the dopamine D2 receptors.
Haloperidol is such an antipsychotic drug, which was developed in the 1950s and entered the clinic soon after that. Its use is limited by the high incidence of extrapyramidal symptoms (movement disorders caused by drugs affecting the extrapyramidal system, a neural network which is part of the motor system). Nevertheless, haloperidol may be used for the rapid control of hyperactive psychotic states and is popular for treating restlessness in the elderly. | [Biological Activity]
Dopamine antagonist with selectivity for D 2 -like receptors (K i values are 1.2, ~ 7, 2.3, ~ 80 and ~ 100 nM for D 2 , D 3 , D 4 , D 1 and D 5 receptors respectively). Subtype-selective NMDA antagonist. | [Biochem/physiol Actions]
Haloperidol is a butyrophenone antipsychotic. It is also classified as a neuroleptic (powerful tranquilizer). Haloperidol acts as a D2, D3, and D4 dopamine receptor antagonist and thus causes Parkinson′s disorder. It also has a negative effect on the central nervous system. | [Clinical Use]
Sedative in severe anxiety
Intractable hiccup
Motor tics
Nausea and vomiting
Schizophrenia and other psychoses | [Synthesis]
Haloperidol, 4-[4-(p-chlorophenyl)-4-hydroxypiperidino]-4??-fluorobutyrophe�none (6.3.8), is synthesized by the alkylation of 4-(4-chlorophenyl)-4-hydroxypiperidine
(6.3.7) using 4??-chloro-4-fluorobutyrophenone (6.3.4). 4-(4-Chlorophenyl) -4-hydroxypiperi�dine (6.3.7) is synthesized from 2-(4-chlorophenyl)propene, which on reaction with formalde�hyde and ammonium chloride gives the intermediate 4-methyl-4-(4-chlorophenyl)-1,
3-oxazine (6.3.5), evidently through stages postulated for the Prince reaction. Treatment of the
resulting product with hydrochloric acid leads to the formation of 4-(4-chlorophenyl)-1,2,3,6-
tetrahydropiperidine (6.3.6), probably through a stage of opening of the hydrogenated
1,3-oxazine ring, followed by dehydration, and subsequent recyclization. Addition of hydro�gen bromide to the double bond of 4-(4-chlorophenyl)1,2,3,6-tetrahydropipidine (6.3.6) and
the subsequent alkaline hydrolysis of the 4-(4-chlorophenyl)-4-bromopiperidine formed dur�ing the reaction, gives 4-(4-chlorophenyl)-4-hydroxypiperidine (6.3.7), the reaction of which
with 4??-chloro-4-fluorobutyrophenone (6.3.4) gives the desired haloperidol (6.3.6) [41¨C46].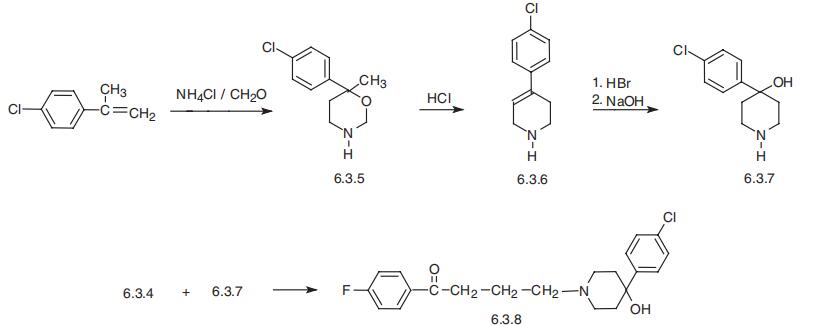
| [Drug interactions]
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effects.
Analgesics: increased risk of convulsions with
tramadol; enhanced hypotensive and sedative
effects with opioids; possibly severe drowsiness
with indometacin or acemetacin; increased risk of
ventricular arrhythmias with methadone.
Anti-arrhythmics: increased risk of ventricular
arrhythmias with anti-arrhythmics that prolong
the QT interval; increased risk of ventricular
arrhythmias with amiodarone or disopyramide -
avoid.
Antibacterials: increased risk of ventricular
arrhythmias with moxifloxacin and delamanid -
avoid with moxifloxacin; concentration reduced by
rifampicin.
Antidepressants: increased risk of ventricular
arrhythmias with citalopram, escitalopram and
tricyclics - avoid; concentration increased by
fluoxetine and venlafaxine and possibly fluvoxamine;
possible increased risk of convulsions with
vortioxetine; concentration of tricyclics increased.
Antiepileptics: metabolism increased by
carbamazepine, phenobarbital and primidone;
lowered seizure threshold; concentration reduced by
fosphenytoin and phenytoin.
Antifungals: concentration possibly increased by
itraconazole.
Antimalarials: avoid with artemether/lumefantrine
and piperaquine with artenimol; possible increased risk of ventricular arrhythmias with mefloquine or
quinine - avoid.
Antipsychotics: avoid concomitant use of depot
formulations with clozapine (cannot be withdrawn
quickly if neutropenia occurs); increased risk
of ventricular arrhythmias with sulpiride and
droperidol and possibly risperidone - avoid with
droperidol; concentration possibly increased by
chlorpromazine.
Antivirals: concentration possibly increased with
ritonavir; increased risk of ventricular arrhythmias
with saquinavir - avoid.
Anxiolytics and hypnotics: increased sedative
effects; concentration increased by alprazolam and
buspirone.
Atomoxetine: increased risk of ventricular
arrhythmias.
Beta-blockers: increased risk of ventricular
arrhythmias with sotalol.
Cytotoxics: increased risk of ventricular arrhythmias
with bosutinib, ceritinib and vandetanib - avoid with vandetanib; increased risk of ventricular arrhythmias
with arsenic trioxide.
Lithium: increased risk of extrapyramidal side effects
and possibly neurotoxicity. | [Metabolism]
Haloperidol is metabolised in the liver and is excreted in
the urine and, via the bile in the faeces; there is evidence
of enterohepatic recycling. Routes of metabolism of
haloperidol include oxidative N-dealkylation, particularly
via the cytochrome P450 isoenzymes CYP3A4 and
CYP2D6, glucuronidation, and reduction of the ketone
group to form an alcohol known as reduced haloperidol.
Metabolites are ultimately conjugated with glycine
and excreted in the urine. There is debate over the
pharmacological activity of the metabolites. | [Dosage forms]
Dosage for haloperidol is as follows:
? Sedation: 2–10 mg i.v. or i.m. (max. 18 mg per 24 h).
? Antiemesis: 1.25 mg i.v. for prevention of postoperative
nausea and vomiting (PONV). | [References]
[1] dr ananya mandal, md .haloperidol pharmacology. |
|
|