| Identification | More | [Name]
DROPERIDOL | [CAS]
548-73-2 | [Synonyms]
1-(1-[4-FLUOROBENZOYLPROPYL]-1,2,3,6-TETRAHYDRO-4-PYRIDYL)-2-BENZIMIDAZOLINONE
1-[1-(P-FLUOROBENZOYLPROPYL)-1,2,3,6-TETRAHYDRO-4-PYRIDYL]-2-BENZIMIDAZOLINONE
DROPERIDOL
1-(1-(3-(p-fluorobenzoyl)propyl)-1,2,3,6-tetrahydro-4-pyridyl)-2-benzimidazo
1-(1-(3-(p-Fluorobenzoyl)propyl)-1,2,3,6-tetrahydro-4-pyridyl)-2-benzimidazolinone
1-(1-(4-(4-fluorophenyl)-4-oxobutyl)-1,2,3,6-tetrahydro-2h-benzimidazol-2-on
1-(1-(4-(p-fluorophenyl)-4-oxobutyl)-1,2,3,6-tetrahydro-4-pyridyl)-2-benzimi
1-(1-(4-(p-Fluorophenyl)-4-oxobutyl)-1,2,3,6-tetrahydro-4-pyridyl)-2-benzimidazolinone
1-(1-(4-(p-fluorophenyl-4-oxobutyl)-1,2,3,6-tetrahydro-4-pyridyl)-2-benzimidaz
1-(4-Fluorophenyl)-4-(4-(2-hydroxy-1H-benzimidazol-1-yl)-3,6-dihydro-1(2H)-pyridinyl)-1-butanone
1-{1-[3-(p-Fluorobenzoyl)propyl]-1,2,3,6-tetrahydro-4-pyridyl}-2-benzimidazolinone
2-Benzimidazolinone, 1-[1-[3-(p-fluorobenzoyl)propyl]-1,2,3,6-tetrahydro-4-pyridyl]-
2-benzimidazolinone,1-(1-(3-(p-fluorobenzoyl)propyl)-1,2,3,6-tetrahydro-4-pyri
2H-Benzimidazol-2-one, 1-[1-[4-(4-fluorophenyl)-4-oxobutyl]-1,2,3,6-tetrahydro-4-pyridinyl]-1,3-dihydro-
3-dihydro-4-pyridinyl)-
component of Innovar
component of Thalamonal
Dehidrobenzperidol
Dehydrobenzperidol
Deidrobenzperidolo | [EINECS(EC#)]
208-957-8 | [Molecular Formula]
C22H22FN3O2 | [MDL Number]
MFCD00083290 | [Molecular Weight]
379.43 | [MOL File]
548-73-2.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed. | [Safety Statements ]
S36:Wear suitable protective clothing . | [RIDADR ]
3249 | [WGK Germany ]
3
| [RTECS ]
DE2100000
| [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
2933995800 | [Hazardous Substances Data]
548-73-2(Hazardous Substances Data) | [Toxicity]
LD50 oral in rat: 750mg/kg |
| Hazard Information | Back Directory | [Description]
D roperidol is a butyrophenone that has potent antidopaminergic (D2)
activity and mild α2-blocking actions. It produces sedation and anxiolysis and
is an effective antiemetic. A dverse effects include
vasodilatation and hypotension, and at higher doses, dystonic reactions can
occur. D roperidol was used for premedication and in neuroleptanaesthesia
until reports of death from long QT syndrome led to its withdrawal in 2001. It
has recently been reintroduced and licenced at lower doses for prevention of
postoperative nausea and vomiting. D roperidol has an onset of 3–10 min
after i.v. injection and duration of action of 6–12h. It undergoes hepatic
metabolism, but approximately 10% of the drug is excreted unchanged in the
urine. | [Chemical Properties]
Pale Yellow Solid | [Originator]
Dehydrobenzperidol,Janssen,W. Germany,1963 | [Uses]
A D1, D2 dopamine receptor antagonist; butyrophenone antipsychotic and anti-emetic. | [Uses]
A D1DR and D2DR inhibitor. | [Uses]
H2 antihistamine | [Uses]
The neuroleptic droperidol possesses antipsychotic, sedative, and antishock action. It poten�tiates the action of drugs for narcosis. In psychiatric practice, droperidol is used for psy�chomotor excitement and hallucinations. The principal use of this drug lies in anesthesiology
for neuroleptanalgesia in combination with fentanyl. It is used in premedication as well as in
surgical operations and post-operational circumstances. | [Definition]
ChEBI: An organofluorine compound that is haloperidol in which the hydroxy group has been eliminated with the introduction of a double bond in the piperidine ring, and the 4-chlorophenyl group has been replaced by a benzimidazol-2-on-1-yl group. It is used in the
management of chemotherapy-induced nausea and vomiting, and in conjunction with an opioid analgesic such as fentanyl to maintain the patient in a calm state of neuroleptanalgesia with indifference to surroundings but still able to cooperate with the surgeo
. | [Manufacturing Process]
A mixture of 10 parts of γ-chloro-4-fluorobutyrophenone, 5.5 parts of 1-
(1,2,3,6-tetrahydro-4-pyridyl)-2-benzimidazolinone, 4 parts of sodium
carbonate, and 0.1 part of potassium iodide in 176 parts of 4-methyl-2-
pentanone is stirred and refluxed for 64 hours. The cooled reaction mixture is
filtered and the solvent is evaporated from the filtrate to leave an oily residue
which is dissolved in toluene. The toluene solution is filtered and the solvent is
evaporated. The resultant residue is recrystallized from a mixture of 32 parts
of ethyl acetate and 32 parts of diisopropyl ether to give 1-[1-[(4-fluorobenzoyl)propyl]-1,2,3,6-tetrahydro-4-pyridyl]-2-benzimidazolinone
hydrate melting at about 145°-146.5°C. | [Brand name]
Inapsine (Akorn);Dehydrobenzperidol;Diaperidol;Inopsin. | [Therapeutic Function]
Tranquilizer | [General Description]
Droperidol, 1-{1-[3-(p-fluorobenzoyl)propyl]-1,2, 3,6-tetrahydro-4-pyridyl}-2-benzimidazolinone(Inapsine), may be used alone as a preanestheticneuroleptic or as an antiemetic. Because of its very shortactingand highly sedating properties, its most frequent useis in combination (Innovar) with the narcotic agent fentanyl(Sublimaze) preanesthetically. | [General Description]
Droperidol, 1-1-[3-(p-fluorobenzoyl)propyl]-1,2,[3,6-tetrahydro-4-pyridyl]-2-benzimidazolinone(Inapsine). Centrally acting acetylcholinesteraseinhibitors may increase the risk of antipsychotic-relatedEPS. CNS depressants may produce additive sedativeeffects (benzodiazepines, barbiturates, antipsychotics,ethanol, opiates, and other sedative medications).Droperidol in combination with certain forms of inhalationanesthetics may produce peripheral vasodilatation and hypotension.Metoclopramide may increase the risk of EPSproduced by droperidol. | [Biochem/physiol Actions]
D1, D2 dopamine receptor antagonist; butyrophenone antipsychotic and anti-emetic. | [Clinical Use]
#N/A | [Synthesis]
Droperidol, 1-[1-[3-(p-fluorobenzoyl)propyl]-1,2,3,6,4-piridyl]-2-benzymi�dazolinone (6.3.11), is synthesized from 1-benzyl-3-carbethoxypiperidin-4-one (3.1.47),
which is reacted with o-phenylendiamine. Evidently, the first derivative that is formed
under the reaction conditions, 1,5-benzdiazepine, rearranges into 1-(1-benzyl-1,2,3,6-
tetrahydro-4-piridyl)-2-benzymidazolone (6.3.9). Debenzylation of the resulting product
with hydrogen over a palladium catalyst into 1-(1,2,3,6-tetrahydro-4-piridyl)-2-benzimi�dazolon (6.3.10) and subsequent alkylation of this using 4??-chloro-4-fluorobutyrophenone
(6.3.4) yields droperidol (6.3.11) [47¨C49].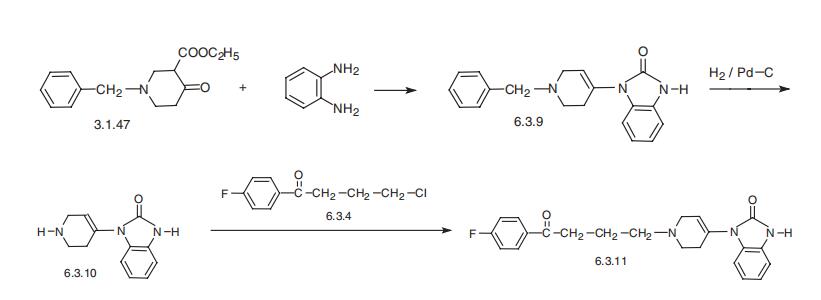
| [Drug interactions]
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect; effects of
thiopental enhanced.
Analgesics: increased risk of ventricular arrhythmias
with methadone; increased risk of convulsions with
tramadol; enhanced hypotensive and sedative effects
with opioids.
Anti-arrhythmics increased risk of ventricular
arrhythmias with anti-arrhythmics that prolong
the QT interval, e.g. procainamide, disopyramide,
dronedarone and amiodarone - avoid.
Antibacterials: increased risk of ventricular
arrhythmias with moxifloxacin and macrolides -
avoid; increased risk of ventricular arrhythmias with
delamanid.
Antidepressants: increased risk of ventricular
arrhythmias with fluoxetine, fluvoxamine, sertraline
or tricyclics - avoid; possible increased risk of
convulsions with vortioxetine.
Antiepileptics: convulsive threshold lowered.
Antimalarials: avoid with artemether/lumefantrine
and piperaquine with artenimol; increased risk
of ventricular arrhythmias with chloroquine,
hydroxychloroquine or quinine - avoid.
Antipsychotics: increased risk of ventricular
arrhythmias with amisulpride, pimozide, sulpiride,
phenothiazines that prolong QT interval or
haloperidol - avoid; possibly increased risk of
ventricular arrhythmias with risperidone.
Antivirals: concentration possibly increased with ritonavir
Anxiolytics and hypnotics: increased sedative effects.
Atomoxetine: increased risk of ventricular
arrhythmias.
Beta-blockers: enhanced hypotensive effect;
increased risk of ventricular arrhythmias with sotalol
- avoid.
Cytotoxics: increased risk of ventricular arrhythmias
with arsenic trioxide and possibly ceritinib.
Desferrioxamine: avoid concomitant use.
Diuretics: enhanced hypotensive effect.
Hormone antagonists: increased risk of ventricular
arrhythmias with tamoxifen - avoid.
Lithium: increased risk of extrapyramidal side effects
and possibly neurotoxicity.
Pentamidine: increased risk of ventricular
arrhythmias - avoid.
Tacrolimus: increased risk of ventricular arrhythmias
- avoid. | [Metabolism]
Extensively metabolised in the liver, and undergoes
oxidation, dealkylation, demethylation and hydroxylation
by cytochrome P450 isoenzymes 1A2 and 3A4, and to a
lesser extent by 2C19. The metabolites are inactive.
About 75% of a dose is excreted in the urine, with 1%
being excreted unchanged; 11% appears in the faeces. |
|
|