| Identification | More | [Name]
Sulfuric acid | [CAS]
7664-93-9 | [Synonyms]
ACID DETERGENT
AMINE-SULFURIC ACID REAGENT
METHANOL/SULFURIC ACID
Acide sulfurique
acidesulfurique
acidesulfurique(french)
Acido solforico
acidosolforico
acidosulfurico
caswellno815
dihydrogensulfate
Dipping acid
dippingacid
Electrolyte acid
electrolyteacid
epapesticidechemicalcode078001
Hydgogen sulfate
Hydrogen sulfate
Inorganic acid
Matting acid | [EINECS(EC#)]
231-639-5 | [Molecular Formula]
H2O4S | [MDL Number]
MFCD00282010 | [Molecular Weight]
98.08 | [MOL File]
7664-93-9.mol |
| Chemical Properties | Back Directory | [Appearance]
Sulfuric acid is a colorless to dark brown, odorless, oily liquid which is commercially sold @ 93% to 98% H2SO4, the remainder being water. | [Melting point ]
10°C | [Boiling point ]
~290 °C (lit.) | [density ]
1.840 g/mL at 25 °C(lit.)
| [vapor density ]
<0.3 (25 °C, vs air)
| [vapor pressure ]
1 mm Hg ( 146 °C)
| [Fp ]
11 °C | [storage temp. ]
Store at RT. | [solubility ]
H2O: soluble
| [form ]
Viscous Liquid | [pka]
-3-2(at 25℃) | [color ]
Pale yellow to slight tan | [Specific Gravity]
1.84 | [Odor]
Odorless | [PH]
2.75(1 mM solution);1.87(10 mM solution);1.01(100 mM solution); | [Stability:]
Stable, but reacts with moisture very exothermically, which may enhance its ability to act as an oxidizing agent. Substances to be avoided include water, most common metals, organic materials, strong reducing agents, combustible materials, bases, oxidising agents. Reacts violently with water-when diluting concentrated acid, carefully and slo | [Water Solubility ]
miscible | [Sensitive ]
Hygroscopic | [Merck ]
14,8974 | [Dielectric constant]
84.0(20℃) | [Exposure limits]
TLV-TWA air 1 mg/m3 (ACGIH, MSHA,
and OSHA); TLV-STEL 3 mg/m3 (ACGIH).
. | [LogP]
-1 at 25℃ | [Uses]
Sulfuric Acid is an acidulant that is a clear, colorless, odorless liquid
with great affinity for water. it is prepared by reacting sulfur dioxide
with oxygen and mixing the resulting sulfur trioxide with water, or
by reacting nitric oxide with sulfur dioxide in water. it is very cor-
rosive. it is used as a modifier of food starch and is used in caramel
production and in alcoholic beverages. | [CAS DataBase Reference]
7664-93-9(CAS DataBase Reference) | [NIST Chemistry Reference]
Sulfuric acid(7664-93-9) | [EPA Substance Registry System]
7664-93-9(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
C,T,F,Xi | [Risk Statements ]
R36/38:Irritating to eyes and skin .
R35:Causes severe burns.
R39/23/24/25:Toxic: danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed .
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed .
R11:Highly Flammable. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S30:Never add water to this product .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36/37:Wear suitable protective clothing and gloves .
S16:Keep away from sources of ignition-No smoking . | [RIDADR ]
UN 3264 8/PG 3
| [WGK Germany ]
1
| [RTECS ]
WS5600000
| [F ]
3 | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
II | [HS Code ]
28070010 | [Safety Profile]
Suspected human carcinogen when contained in strong inorganic mists. A human poison. Experimental poison by inhalation. Moderately toxic by ingestion. A severe eye irritant. Extremely irritating, corrosive, and toxic to tissue, resulting in rapid destruction of tissue, causing severe burns. If much of the skin is involved, exposure is accompanied by shock, collapse, and symptoms similar to those seen in severe burns. Repeated contact with dilute solutions can cause a dermatitis, and repeated or prolonged inhalation of a mist of sulfuric acid can cause inflammation of the upper respiratory tract, leading to chronic bronchitis. Sensitivity to sulfuric acid or its mists or vapors varies with individuals. Normally 0.125-0.50 ppm may be mildly annoying, 1.5-2.5 ppm can be definitely unpleasant, and 10-20 ppm is unbearable. Workers exposed to low concentrations of the vapor gradually lose their sensitivity to its irritating action. Inhalation of concentrated vapor or mists from hot acid or oleum can cause rapid loss of consciousness with serious damage to lung tissue. Severe exposure may cause a chemical pneumonitis; erosion of the teeth due to exposure to strong acid fumes has been recopzed in industry. An experimental teratogen.Ths is a very powerful acidc oxidmer that can ignite or explode on contact with many materials, e.g., acetic acid, acetone cyanhydrin, (acetone + HNO3), (acetone + K2Cr207), acetonitrile, acrolein, acrylonitrile, (acrylonitrile + H20), (alcohols + H2O2), allyl alcohol, allyl chloride, NH4OH, 2-amino ethanol, NH4, triperchromate, anhe, (bromates + metals), BrF5, n-butyraldehyde, carbides, CoHC2, chlorates, (metals + chlorates), ClF3, chlorosulfonic acid, Cu3N, diisobutylene, (dimethyl benzylcarbinol + H204, epichlorohydrin, ethylene cyanhydrin, ethylene diamine, ethylene glycol, ethylene imine, fulminates, HCl, H2, IF7, (indene + HNO3), Fe, isoprene, LisSiz, Hg3N2, mesityl oxide, metals, (HNO3 + glycerides), p-nitrotoluene, perchlorates, HClO4, (C6H6 + permanganates), pentasilver trihydroxydiamino phosphate, (l-phenyl-2-methyl propyl alcohol + H2O2), P, P(OCN)3, picrates, potassium-tertbutoxide, KClO3, KMnO4 + KCl), (KMnO4 + H2O), p-propiolactone, RbHC2, propylene oxide, pyridine, Na, Na2CO3, NaOH, steel, styrene monomer, water, vinyl acetate, (HNO3 + toluene). When heated it emits highly toxic fumes; wdl react with water or steam to produce heat; can react with oxidizing or reducing materials. When heated to decomposition it emits toxic fumes of SOX. See also SULFATES. | [Hazardous Substances Data]
7664-93-9(Hazardous Substances Data) | [Toxicity]
LD50 orally in rats: 2.14 g/kg (Smyth) | [IDLA]
15 mg/m3 |
| Hazard Information | Back Directory | [Reactivity Profile]
SULFURIC ACID is strongly acidic. Reacts violently with bromine pentafluoride [Mellor 2 Supp. 1:172 1956]. Exploded with para-nitrotoluene at 80 °C [Chem. Eng. News 27:2504]. An explosion occurred when concentrated sulfuric acid was mixed with crystalline potassium permanganate in a vessel containing moisture. Manganese heptoxide was formed, which explodes at 70°C [Delhez 1967]. A mixture of acrylonitrile with concentrated sulfuric acid must be kept well chilled, otherwise a vigorous exothermic reaction occurs [Chem. Safety Data Sheet SD-31:8. 1949]. Mixing sulfuric acid (96%) in equal portions with any of the following substances in a closed container caused the temperature and pressure to increase: acetonitrile, acrolein, 2-aminoethanol, ammonium hydroxide (28%), aniline, n-butyraldehyde, chlorosulfonic acid, ethylene diamine, ethyleneimine, epichlorohydrin, ethylene cyanohydrin, hydrochloric acid (36%), hydrofluoric acid (48.7%), propiolactone, propylene oxide, sodium hydroxide, styrene monomer [NFPA 1991]. Sulfuric acid (concentrated) is extremely hazardous in contact with carbides, bromates, chlorates, fulminates, picrates, and powdered metals [Haz. Chem. Data 1966]. Allyl chloride may polymerize violently under conditions involving an acid catalyst, such as sulfuric acid [Ventrone 1971]. React exothermically with sodium hypochlorite to produce chlorine gas. Mixing chlorosulfuric acid and 98% sulfuric acid may evolve HCl [Subref: Anon, Loss Prev. Bull. 1977, (013), 2-3]. Zinc iodide reacts violently with H2SO4. (Pascal, 1962, Vol. 5, 168). | [Air & Water Reactions]
Reaction with water is negligible unless acid strength is above 80-90% then heat from hydrolysis is extreme, may cause severe burns [Merck, 11th ed. 1989]. During sulfonation of mononitrobenzene by fuming sulfuric acid, a leak from an internal cooling coil permitted water to enter the reaction tank. A violent eruption occurred due to the heat of solution [MCA Case History 944 1963]. | [Health Hazard]
Corrosive to all body tissues. Inhalation of vapor may cause serious lung damage. Contact with eyes may result in total loss of vision. Skin contact may produce severe necrosis. Fatal amount for adult: between 1 teaspoonful and one-half ounce of the concentrated chemical. Even a few drops may be fatal if the acid gains access to the trachea. Chronic exposure may cause tracheobronchitis, stomatitis, conjunctivitis, and gastritis. Gastric perforation and peritonitis may occur and may be followed by circulatory collapse. Circulatory shock is often the immediate cause of death. Those with chronic respiratory, gastrointestinal, or nervous diseases and any eye and skin diseases are at greater risk. | [Potential Exposure]
Used as a chemical feedstock in the manufacture of acetic acid, hydrochloric acid; citric acid; phosphoric acid; aluminum sulfate; ammonium sulfate;barium sulfate; copper sulfate; phenol, superphosphates, titanium dioxide; as well as synthetic fertilizers, nitrate explosives; artificial fibers; dyes, pharmaceuticals, detergents, glue, paint, and paper. It finds use as a dehydrating agent for esters and ethers due to its high affinity for water; as an electrolyte in storage batteries; for the hydrolysis of cellulose to obtain glucose; in the refining of mineral and vegetable oil; and in the leather industry. Other uses include fur and food processing; carbonization of wool fabrics; gas drying; uranium extraction from pitchblende; and laboratory analysis. Sulfuric acid is among the highestvolume produced chemical in the United States. | [Fire Hazard]
SULFURIC ACID is highly reactive and capable of igniting finely-divided combustible materials on contact. When heated, SULFURIC ACID emits highly toxic fumes. Avoid heat; water and organic materials. Sulfuric acid is explosive or incompatible with an enormous array of substances. Can undergo violent chemical change at elevated temperatures and pressure. May react violently with water. When heated, SULFURIC ACID emits highly toxic fumes. Hazardous polymerization may not occur. | [First aid]
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 minutes, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water, or milk. Do not induce vomiting. Medical observation is recommended for 24 to 48 hours after breathing overexposure, as pulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consider administering a drug or other inhalation therapy. | [Shipping]
UN1830 Sulfuric acid with >51% acid or sulfuric acid with not >51% acid, Hazard class: 8; Labels: 8-Corrosive material. UN1831 Sulfuric acid, fuming with 30% or more free sulfur trioxide and Sulfuric acid, fuming, with <30% free sulfur trioxide, Hazard class: 8; Labels: 8-Corrosive material. UN1832 Sulfuric acid, spent, Hazard class: 8; Labels: 8-Corrosive material. | [Incompatibilities]
A strong acid and oxidizer. Reacts violently with water with dangerous spattering and evolution of heat. Reacts violently with combustible and reducing materials; bases, organic materials; chlorates, carbides, picrates, fulminates, water, powdered metals. Corrosive to most common metals forming explosive hydrogen gas. | [Description]
Reactivity
Sulfuric acid is very reactive and dissolves most metals, it is
a concentrated acid that oxidizes, dehydrates, or sulfonates
most organic compounds, often causes charring.
Sulfuric acid reacts violently with alcohol and water to release
heat. It reacts with most metals, particularly when diluted with
water, to form flammable hydrogen gas, which may create an
explosion hazard. Sulfuric acid is not combustible, but it is
a strong oxidizer that enhances the combustion of other substances,
does not burn itself. During fire, poisonous gases are
emitted. Hazardous decomposition products are as follows:
sulfur dioxide, sulfur trioxide, and sulfuric acid fumes.
Note: Use great caution in mixing with water due to heat
release that causes explosions. Always add the acid to water,
never the reverse.
Where Found
l Car battery acid
l Certain detergents
l Chemical munitions
l Some fertilizers
l Some toilet bowl cleaners
Derivation
Sulfuric acid is made from sulfur, pyrite (FeS2), hydrogen
sulfide, or sulfur-containing smelter gases by the contact process
(vanadium pentoxide catalyst). The first step is combustion of
elemental sulfur, or roasting of iron pyrites, to yield sulfur
dioxide. Then follows the critical reaction, catalytic oxidation of
sulfur dioxide to sulfur trioxide. | [Waste Disposal]
Add slowly to solution of soda ash and slaked lime with stirring; flush to drain with large volumes of water. Recovery and reuse of spent sulfuric acid may be a viable alternative to disposal, and processes are available. | [Definition]
Sulfuric acid,H2S04, also known as oil of vitriol and dipping acid,is a colorless, toxic,oily liquid.A great deal of heat is released when concentrated sulfuric acid and water are mixed;therefore, acid should always be added to water with sufficient stirring to prevent splattering and boiling. Sulfuric acid has a strong attraction for water and forms four crystalline hydrates. This affinity for water makes sulfuric acid an efficient drying agent for gases such as hydrogen, oxygen,nitrogen, and carbon dioxide,but results in the charring of organic compounds containing carbon,hydrogen, and oxygen such as cellulose, sugar,paper, and wood. Sulfuric acid participates in two types of oxidation reactions. One is the typical reaction of a strong acid that depends on the oxidizing power of the hydrogen ion, for example, the reaction of an active metal with the dilute acid to produce hydrogen. Sulfuric acid is a strong electrolyte and is used in electroplating baths,for pickling, and for other operations in the production of iron and steel. In the second type of oxidation reaction, the sulfate portion of the molecule reacts to form acid sulfates or bisulfates and the normal sulfates. Sulfuric acid is used in the manufacture of fertilizers, organic pigments, explosives, rayon, and film, Sulfuric acid has low volatility, a feature utilized in the manufacture of volatile acids such as nitric, hydrochloric, and hydrofluoric, where the volatile acid is vaporized when one of its salts is heated with the sulfuric acid.
| [Production Methods]
Sulfuric acid may be prepared industrially by either the contact process or the chamber process.
Contact Process
2SO2+O2→2SO3
SO3+H2O→H2SO4
Chamber Process
2NO+O2→2NO2
NO2+SO2+H2O→H2SO4+NO
| [General Description]
Sulphuric acid may be prepared by catalytic oxidation of sulphur dioxide. It is a very strong electrolyte and has high affinity to water. | [Hazard]
Strong irritant to tissue. Pulmonary function
inhibitor. Confirmed carcinogen. | [Flammability and Explosibility]
Sulfuric acid is noncombustible but can cause finely divided combustible substances
to ignite. Sulfuric acid reacts with most metals, especially when dilute, to produce
flammable and potentially explosive hydrogen gas. | [Pharmaceutical Applications]
Sulfuric acid is used as an acidifying agent in a variety of
pharmaceutical and food preparations. It may also be used to
prepare dilute sulfuric acid, which, in addition to its use as an
excipient, has some therapeutic use for the treatment of gastric
hypoacidity, as an astringent in diarrhea, or to stimulate appetite.
Sulfuric acid has been used in parenteral, oral, topical, and
ophthalmic pharmaceutical formulations. | [Industrial uses]
Sulfuric acid (H2SO4) is the most widely used acid for pH control in mineral flotation.
Sulfuric acid can be manufactured by several processes including the burning of pure
sulfur, roasting of pyrite and from the recovery of SO2 stack gas from a smelter operation.
Sulfuric acid is a colorless to amber, slightly cloudy and oily liquid with a specific
gravity of 1.84 at 95% strength.
In mineral flotation, sulfuric acid is used in almost all applications involving acid pH
control. It is also used as a pulp pretreatment chemical during flotation of oxidic and
industrial minerals. Pulp pretreatment with sulfuric acid improves flotation of ilmenite,
perovskite, phenacite, beryl and other minerals. | [Safety]
Sulfuric acid is widely used in a variety of pharmaceutical
formulations. Although concentrated sulfuric acid is very corrosive,
it is normally used well diluted in formulations. Concentrated
sulfuric acid will react violently with water and much heat is
generated. When diluting sulfuric acid, the acid should always be
added to the other liquid with great caution.
The concentrated solution is extremely corrosive and can cause
severe damage or necrosis on contact with the eyes and skin.
Ingestion may cause severe injury or death. Inhalation of
concentrated vapors can cause serious lung damage.
LD50 (rat, oral): 2.14 g/kg | [Carcinogenicity]
Strong inorganic acid mists containing sulfuric acid are known to be human carcinogens based on sufficient evidence of carcinogenicity
from studies in humans. | [Environmental Fate]
Although sulfuric acid can be extremely harmful, it is a naturally
occurring compound. The release of sulfur into the
biosphere is not from anthropogenic sources. It is also a major
compound that is released in volcanic eruptions when oxides
of sulfur are emitted:
Sulfur trioxide will dissolve in rainwater to form sulfuric
acid
SO3 + H2O → H2SO4:
Sulfur dioxide will dissolve in rainwater to form sulfurous
acid (H2SO3), and is then oxidized to form sulfuric acid,
which leads to acid rains.
The presence of sulfuric acid is related with the natural
ability of microorganisms that can be found in or isolated from
acid mine water or from sulfur and iron sulfide mines as well as
volcanoes.
The examples of such bacteria are:
Acidithiobacillus ferrooxidans (Thiobacillus ferrooxidans) that
lives in pyrite deposits, metabolizing iron and sulfur and
producing sulfuric acid.
Acidithiobacillus thiooxidans (Thiobacillus thiooxidans, Thiobacillus
concretivorus) that utilizes sulfur and produces
sulfuric acid. | [storage]
Splash goggles and rubber gloves should be worn when
handling this acid, and containers of sulfuric acid should be stored in a wellventilated
location, separated from organic substances and other combustible
materials. Containers of sulfuric acid should be stored in secondary plastic trays to
avoid corrosion of metal storage shelves due to drips or spills. Water should never
be added to sulfuric acid because splattering may result; always add acid to water | [storage]
Sulfuric acid is stable but very corrosive and hygroscopic. It will
draw moisture from the atmosphere. Sulfuric acid should be stored
in a tightly closed container in an explosion-proof area. Containers
should be stored out of direct sunlight and away from heat. Avoid
heat and moisture. Isolate from incompatible materials. | [Purification Methods]
Sulfuric acid, and also 30% fuming H2SO4, can be distilled in an all-Pyrex system, optionally from potassium persulfate. It has been purified by fractional crystallisation of the monohydrate from the liquid. It has a very strong dehydrating action and attacks skin—wash immediately with cold H2O; otherwise the skin can be scarred for life. It is very hygroscopic and has been used as a desiccant in desiccators. Dilution with H2O is highly exothermic, and because the concentrated acid is much more dense than H2O it is diluted by running the concentrated acid down the side of the container of H2O with slowly stirring while cooling the outside of the container. If these precautions are not taken, the H2O is likely to boil vigorously. | [Regulatory Status]
GRAS listed. Accepted for use as a food additive in Europe.
Included in the FDA Inactive Ingredients Database (IM, IV, and IP
injections, inhalation solutions, irrigation solutions, nasal, ophthalmic
solutions and suspensions, oral solutions, and topical emulsions
and creams). Included in nonparenteral and parenteral medicines
licensed in Europe. Included in the Canadian List of Acceptable
Non-medicinal Ingredients.
The United Nations Convention Against Illicit Traffic in
Narcotic Drugs and Psychotropic Substances (1988) lists sulfuric
acid as a chemical frequently used in the illicit manufacture of
narcotic drugs or psychotropic substances. In the USA, sulfuric
acid is included in the list of essential or precursor chemicals
established pursuant to the Chemical Diversion and Trafficking Act.
Accordingly, transactions of sulfuric acid such as imports, exports,
sales, and transfers are subject to regulation and monitoring by the
Drug Enforcement Administration. |
| Questions And Answer | Back Directory | [Chemical structure]
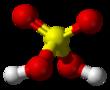
Ball-and-stick diagram
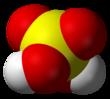
Space-filling model
The chemical formula of sulfuric acid is H2SO4 and its molecular weight is 98.079g/mol. Its chemical structure is shown above. The sulfur atom is bound to two oxygen atoms through double bonds, and two hydroxyl groups (OH) through single bonds. It is a diprotic acid, as it can release two protons. | [History]
Although sulfuric acid is now one of the most widely used chemicals, it was probably little known before the 16th cent. It was prepared by Johann Van Helmont (c.1600) by destructive distillation of green vitriol (ferrous sulfate) and by burning sulfur. The first major industrial demand for sulfuric acid was the Leblanc process for making sodium carbonate (developed c.1790). Sulfuric acid was produced at Nordhausen from green vitriol but was expensive. A process for its synthesis by burning sulfur with saltpeter (potassium nitrate) was first used by Johann Glauber in the 17th cent. and developed commercially by Joshua Ward in England c.1740. It was soon superseded by the lead chamber process, invented by John Roebuck in 1746 and since improved by many others. The contact process was originally developed c.1830 by Peregrine Phillips in England; it was little used until a need for concentrated acid arose, particularly for the manufacture of synthetic organic dyes.
| [Occurrence]
Sulfuric acid is formed naturally by oxidation of sulfide minerals in rocks. Dilute sulfuric acid is also formed in the atmosphere by oxidation of sulfur dioxide (from burning of fuels) in the presence of moisture, eventually precipitating as 'acid rain'.
| [Psysical properties]

H2SO4 is a colorless or slightly yellow viscous liquid with a pungent odor. It has a density of 1.84 g/mL, boiling point of 337 °C, and melting point of 10 °C. "Concentrated" sulfuric acid is 98% in water, and is the most stable form. Many other concentrations, with different names, are available for various purposes. Battery acid is 29–32%, chamber acid is 62-70%, and tower acid is 78-80%. | [Chemical properties]
Sulfuric acid is a very strong, diprotic acid. It is hygroscopic and readily absorbs moisture from air. It is a powerful oxidizing agent and reacts with many metals at high temperatures. Concentrated H2SO4 is also a strong dehydrating agent. Addition of water to concentrated sulfuric acid is a very exothermic reaction and can lead to explosions.
C(s) + H2SO4(aq) → 2SO2(g)+2H2O(l)
Fe(s) + H2SO4(aq) → H2(g) + FeSO4(aq)
Sn(s) + 2 H2SO4(aq) → SnSO4(aq) + 2 H2O(l) + SO2(g)
Fig.2 Oxidizing ability of sulfuric acid

Fig.3 Dehydrating nature of sulfuric acid

Fig.4 Reaction of sulfuric acid with water | [Uses]
Industry
Application
Role/benefit
Chemical manufacture
Manufacture of hydrochloric acid, nitric acid, phosphoric acid and many other industrial chemicals
Raw material
Fertilizers
Manufacture of ammonium sulfate and aluminium sulfate
Raw material
Hydrogen
Sulfur-iodine cycle for hydrogen production
Raw material/ no requirement of hydrocarbons
Cleaning
Removing oxidation, rust and scaling from rolled sheet and billets
Cleaning agent
Preparation of piranha solution (a powerful cleaning solution)
Raw material
Acidic drain cleaners
Main ingredient
Nylon manufacture
Catalyzing the conversion of cyclohexanone oxime to caprolactam
Acid catalyst
Petroleum refining
SAAU or sulphuric acid Alkylation Unit
Electrochemistry
Lead-acid batteries
Electrolyte
Medicine
Manufacture of alkylating antineoplastic agents
Raw material
Manufacture of topical ointment called Debacterol
Key ingredient
Others
Potato Harvesting
Spraying solution/helps to dry out the stem
Manufacture of Rayon
Pcocessing reagent
Manufacture of explosives
Component
Production of acid dyes
Raw material/helps to set the color of the dye
| [Annual production of sulfuric acid]
These figures relate to 2011 2012. It was expected that by 2012, the World production would be over 250 million tonnes (mcgroup.com) and 260 million tonnes by 2018 (marketsandmarkets.com) with the upward trend forecasted to at least 2023 (transparencymarketresearch.com).
Tab. 1 Annual production of sulfuric acid (Data estimated from:Merchant Research & Consulting Ltd.)
| [Production methods of sulfuric acid]
Lean Chamber Process
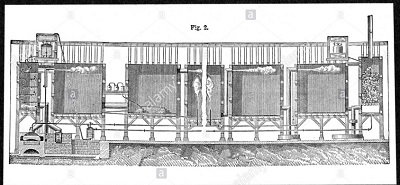
Fig.5 Lead chambers for large scale production of sulphuric acid 1874
In 1746 in Birmingham, England, John Roebuck began producing sulfuric acid in lead-lined chambers, which were stronger and less expensive, and could be made much larger, than the glass containers which had been used previously. This allowed the effective industrialization of sulfuric acid production and, with several refinements, this process remained the standard method of production for almost two centuries. So robust was the process that as late as 1946, the chamber process still accounted for 25% of sulfuric acid manufactured.
Contact Process
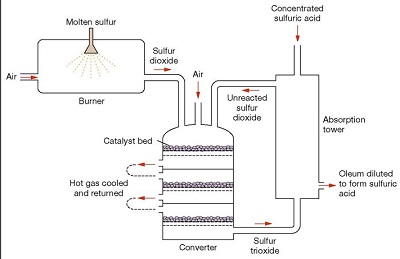
Fig.6 Contact process for producing sulfuric acid
The contact process (DCDA process) is the current method of producing sulfuric acid in the high concentrations needed for industrial processes. This process was patented in 1831 by British vinegar merchant Peregrine Phillips. In addition to being a far more economical process for producing concentrated sulfuric acid than the previous lead chamber process, the contact process also produces sulfur trioxide and oleum. In the contact process, purified sulfur dioxide and air are mixed, heated to about 450°C, and passed over a catalyst; the sulfur dioxide is oxidized to sulfur trioxide. The catalyst is usually platinum on a silica or asbestos carrier or vanadium pentoxide on a silica carrier. The sulfur trioxide is cooled and passed through two towers. In the first tower it is washed with oleum (fuming sulfuric acid, 100% sulfuric acid with sulfur trioxide dissolved in it). In the second tower it is washed with 97% sulfuric acid; 98% sulfuric acid is usually produced in this tower. Waste gases are usually discharged into the atmosphere. Acid of any desired concentration may be produced by mixing or diluting the products of this process.
Wet sulfuric acid process
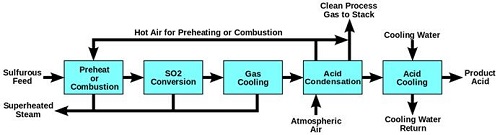
Fig.7 Wet Sulfuric Acid Process Diagram
The wet sulfuric acid process (WSA process) is one of the key gas desulfurization processes on the market today. Since the Danish catalyst company Haldor Topsoe introduced and patented this technology in the late 1980s, it has been recognised as an efficient process for recovering sulfur from various process gasses in the form of commercial quality sulfuric acid (H2SO4), with simultaneous production of high pressure steam. The WSA process is applied in all industries where removal of sulfur is an issue. | [Uses]
Sulfuric acid has many uses in different industries, such as fertilizer production, metal production, mineral processing, petroleum refining, wastewater processing, etc. It is also used in the production of cleaning agents, dyes, pigments, drugs, detergents, and explosives. It is commonly used as the electrolyte in lead-acid batteries.
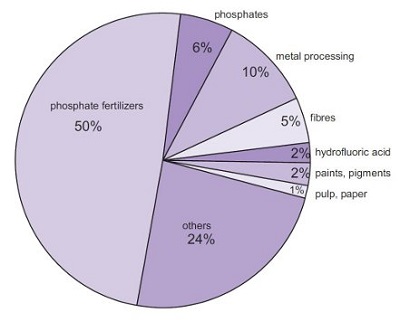
Fig. 8 Uses of sulfuric acid.
Fertilizers
By far the largest amount of sulfuric acid is used to make phosphoric acid, used, in turn, to make the phosphate fertilizers, calcium dihydrogenphosphate and the ammonium phosphates. It is also used to make ammonium sulfate, which is a particularly important fertilizer in sulfur-deficient.
3.2 Industrial cleaning agent
Sulfuric acid is used in large quantities by the iron and steelmaking industry to remove oxidation, rust and scaling from rolled sheet and billets prior to sale to the automobile and major appliances industry. Hydrogen peroxide (H2O2) can be added to sulfuric acid to produce piranha solution, a powerful but very toxic cleaning solution with which substrate surfaces can be cleaned. Piranha solution is typically used in the microelectronics industry, and also in laboratory settings to clean glassware.

Fig. 9 Piranha solution
Metal processing
It is widely used in metal processing for example in the manufacture of copper and the manufacture of zinc and in cleaning the surface of steel sheet, known as 'pickling', prior to it being covered in a thin layer of tin, used to make cans for food.
Catalyst
Sulfuric acid can be used as acid catalysts in many organic reactions. It covers the nitration of benzene, the hydration of ethene to manufacture ethanol, and the reactions both to produce esters and to hydrolyse them under acidic conditions.
Electrolyte
Sulfuric acid acts as the electrolyte in lead–acid batteries (lead-acid accumulator):
At anode:
Pb + SO42− ? PbSO4 + 2 e−
At cathode:
PbO2 + 4 H+ + SO42− + 2 e− ? PbSO4 + 2 H2O
Overall:
Pb + PbO2 + 4 H+ + 2 SO42− ? 2 PbSO4 + 2 H2O

Fig.10 Lead–acid batterie
Domestic uses
Sulfuric acid at high concentrations is frequently the major ingredient in acidic drain cleaners[9] which are used to remove grease, hair, tissue paper, etc. Similar to their alkaline versions, such drain openers can dissolve fats and proteins via hydrolysis. Moreover, as concentrated sulfuric acid has a strong dehydrating property, it can remove tissue paper via dehydrating process as well. Since the acid may react with water vigorously, such acidic drain openers should be added slowly into the pipe to be cleaned.

Fig. 11 Acidic drain cleaner
Others
Fluorapatite is treated with 93% sulfuric acid to produce calcium sulfate, hydrogen fluoride (HF) and phosphoric acid. The HF is removed as hydrofluoric acid. The overall process can be represented as:
Ca5F(PO4)3 + 5 H2SO4 + 10 H2O → 5 CaSO4•2 H2O + HF + 3 H3PO4
Another important use for sulfuric acid is for the manufacture of aluminium sulfate, also known as paper maker's alum.
2 AlO(OH) + 3 H2SO4 → Al2(SO4)3 + 4 H2O
Sulfuric acid is also important in the manufacture of dyestuffs solutions, pigments and drugs. Whatsmore, sulfuric acid can be used in mineral processing, petroleum refining and wastewater processing. | [Toxicity information]
Toxicity Level
High toxicity
Acute Toxicity
Oral-Rat LD50: 2140 mg/kg; Inhalation-mouse LC50: 320 mg /m3/2 h
Hazards
Health hazards
Corrosive to all body tissues. Inhalation of vapor may cause serious lung damage. Contact with eyes may result in total loss of vision. Skin contact may produce severe necrosis. Fatal amount for adult: between 1 teaspoonful and one-half ounce of the concentrated chemical. Even a few drops may be fatal if the acid gains access to the trachea. Chronic exposure may cause tracheobronchitis, stomatitis, conjunctivitis, and gastritis. Gastric perforation and peritonitis may occur and may be followed by circulatory collapse. Circulatory shock is often the immediate cause of death. Those with chronic respiratory, gastrointestinal, or nervous diseases and any eye and skin diseases are at greater risk.
Fire Hazards
Sulfuric acid is highly reactive and capable of igniting finely-divided combustible materials on contact. When heated, Sulfuric acid emits highly toxic fumes. Avoid heat; water and organic materials. Sulfuric acid is explosive or incompatible with an enormous array of substances. Can undergo violent chemical change at elevated temperatures and pressure. May react violently with water. When heated, Sulfuric acid emits highly toxic fumes. Hazardous polymerization may not occur.
| [Storage and transportation]
Sulfuric acid is manufactured by the catalytic oxidation of sulfur dioxide by either the contact process or the lead-chamber process,although the contact process is now the primary process used to manufacture sulfuric acid. Acid produced by the conversion of sulfur trioxide by the contact process is concentrated (98 to 99%) and pure. Because anhydrous sulfuric acid is difficult to ship on account of its high freezing point,10.5°C(50 OF), sulfuric acid is ordinarily shipped at the 93.19% concentration, which is designated as oil of vitriol. This concentration has a low freezing point of -34°C (-29 OF) and does not corrode steel containers at ordinary temperatures.
In the lead-chamber process,sulfur dioxide,oxygen,water vapor, and oxides of nitrogen are mixed in large,lead-lined chambers, and the liquid products as run off from the floor of the chambers is called chamber acid. Chamber acid has a concentration of 60 to 70% sulfuric acid by weight and contains considerable impurities. It reacts with steel shipping containers and is rarely shipped in quantity. Although more dilute acids are prepared, they must be shipped in expensive glass bottles. Chamber acid maybe concentrated to 77% by evaporation in lead-lined pans and shipped in areas where its freezing point of -lO.8 °C(12.6 °F) is acceptable. In cold climates, the concentration may be diluted to 76% to lower the freezing point. Sulfuric acid at the 76 to 78% concentration level is not seriously corrosive to steel tank cars and is an economical supply for the production of superphosphate by the fertilizer industry.
| [Hazards & Safety Information]
Category : Corrosive items
Toxicity classification : high toxic
Acute toxicity :
Oral-Rat LD50: 2140 mg/kg; Inhalation-mouse LC50: 320 mg /m 3/2 h
Stimulation Data : Eye-Rabbit 5 mg/30 sec Severe
Hazardous properties of explosives : exploded after encounter with water; combustion-supporting after encounter with combustible; react with the metal to generate flammable explosive hydrogen
Flammable hazardous nature : burned after encounter with organic matter; release combustible hydrogen after encounter with metal
Storage and transportation characteristics : ventilation; low-temperature; dry; stored separately with organic matter, reducing material and flammable materials
Extinguishing agent : carbon dioxide, dry sand; prohibit the use of columnar water
Occupational Standard : TWA 1 mg /m3; STEL 3 mg /m3 | [References]
http://www.essentialchemicalindustry.org/chemicals/sulfuric-acid.html
https://en.wikipedia.org/wiki/Sulfuric_acid#Uses
http://www.encyclopedia.com/science-and-technology/chemistry/compounds-and-elements/sulfuric-acid
http://www.qsstudy.com/chemistry/uses-sulphuric-acid
|
|