| Identification | More | [Name]
Trimethylsilyl cyanide | [CAS]
7677-24-9 | [Synonyms]
CYANOTRIMETHYLSILANE
CYANTRIMETHYLSILANE
TMSCN
TRIMETHYLCYANOSILANE
TRIMETHYLSILANECARBONITRILE
TRIMETHYLSILYL CYANIDE
TRIMETHYLSILYLNITRILE
trimethyl-silanecarbonitril
trimethylsilycyanide
Cyanotrimethylsilane~TMSCN
Trimethyl Silane Cyanide
trimethylsilylcarbonitrile
Trimethylsilycyanidel
Trimethylsilylcyanide,97%
Trimethylsilylcyanide(Trimethylsilylnitrile)
Silanecarbonitrile, trimethyl-
Trimethylsilyl cyanide (Cyanotrimethylsilane)
Trimethylsilylcyanid
Trimethycyanosilane
TRIMETHYLSILYLCYANIDE (99%) | [EINECS(EC#)]
231-657-3 | [Molecular Formula]
C4H9NSi | [MDL Number]
MFCD00001765 | [Molecular Weight]
99.21 | [MOL File]
7677-24-9.mol |
| Chemical Properties | Back Directory | [Appearance]
clear colorless to yellow liquid | [Melting point ]
8-11 °C(lit.)
| [Boiling point ]
114-117 °C(lit.)
| [density ]
0.793 g/mL at 20 °C(lit.)
| [refractive index ]
n20/D 1.392(lit.)
| [Fp ]
34 °F
| [storage temp. ]
0-6°C | [solubility ]
Miscible with organic solvents. | [form ]
liquid | [color ]
Yellow | [Specific Gravity]
0.744 | [Water Solubility ]
reacts | [Hydrolytic Sensitivity]
8: reacts rapidly with moisture, water, protic solvents | [Sensitive ]
Moisture Sensitive | [Detection Methods]
GC,NMR | [BRN ]
1737612 | [Exposure limits]
NIOSH: IDLH 25 mg/m3 | [Stability:]
Moisture Sensitive | [InChIKey]
LEIMLDGFXIOXMT-UHFFFAOYSA-N | [CAS DataBase Reference]
7677-24-9(CAS DataBase Reference) | [Storage Precautions]
Store under nitrogen;Moisture sensitive | [EPA Substance Registry System]
7677-24-9(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
F,T+,T,N | [Risk Statements ]
R11:Highly Flammable.
R26/27/28:Very Toxic by inhalation, in contact with skin and if swallowed .
R29:Contact with water liberates toxic gas.
R50/53:Very Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment . | [Safety Statements ]
S16:Keep away from sources of ignition-No smoking .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S61:Avoid release to the environment. Refer to special instructions safety data sheet .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [RIDADR ]
UN 3384 6.1/PG 1
| [WGK Germany ]
3
| [F ]
10-21 | [Hazard Note ]
Highly Flammable/Toxic | [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
II | [HS Code ]
29310095 |
| Hazard Information | Back Directory | [Chemical Properties]
Trimethylsilyl cyanide (TMSCN) is a commercial reagent used as cyanide source for nucleophilic reactions. It is a clear colorless to yellow liquid, moisture sensitive, with a boiling point of 114–117 °C, which was first synthetized by the reaction of trimethylsilicon halides TMSX and AgCN in 1952.
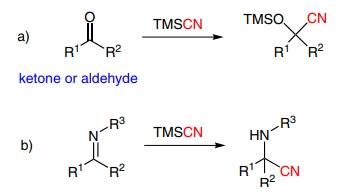
TMSCN is a versatile reagent that can be used in several different reactions, but it is generally used in nucleophilic additions to aldehydes, ketones and imines to form cyanohydrin silyl ethers (Scheme 1a) and α-aminonitriles in Strecker-type reactions (Scheme 1b).
| [Physical properties]
mp 11–12°C; bp 118–119°C; d 0.744 g cm?3. | [Uses]
Cyanotrimethylsilane is a highly versatile
reagent that reacts with a multitude of functional groups to yield an
array of products and/or highly valuable synthetic intermediates.
Aldehydes and ketones are readily transformed into the corresponding
cyanohydrin trimethylsilyl ethers when treated with
cyanotrimethylsilane in the presence of Lewis acids (eq 1),
triethylamine,or solid bases such as CaF2 or hydroxyapatite.
The products can be readily hydrolyzed to the corresponding
cyanohydrins. The cyanosilylation of aromatic aldehydes can
be achieved with high enantioselectivity in the presence of
catalytic amounts of a modified Sharpless catalyst consisting
of titanium tetraisopropoxide and L-(+)-diisopropyl tartrate
(eq 2).Catalysis with chiral titanium reagents yields aliphatic
and aromatic cyanohydrins in high chemical and optical yields (eq 3). Cyanohydrins can be subsequently transformed into a
variety of useful synthetic intermediates (eq 4).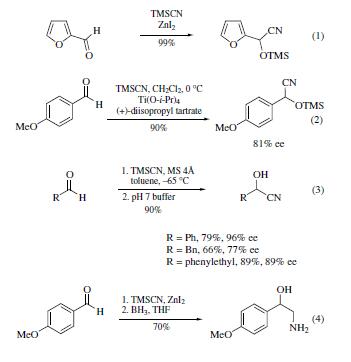
| [Uses]
Reacts with aldehydes and ketones to give cyanohydrin-TMS ethers which can be reduced to β-aminoethyl alcohols.
Trimethylsilyl cyanide (TMSCN) participates in carbonyl aminomethylation via α-silyloxy nitriles.
TMSCN can be used as a reagent in the:
Trimethylsilyl cyanide was used in the second step of an oxidative Michael addition of cyanide anion to Baylis–Hillman adducts. The importance of the addition of cyanide to α,β-unsaturated carbonyl derivatives is that the products can be converted into a variety of compounds including γ-aminobutyric acids. The reaction took place in a liquid ionic medium ([bmim]Br), which was reused several times without losing its activity. The β-cyano carbonyl compounds were obtained with high regioselectivity and yields (>79%).
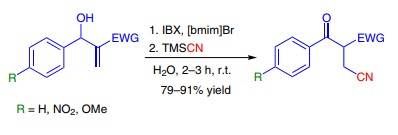
Cyanosilylation of carbonyl compounds using various catalysts.
Synthesis of α-aminonitriles by one-pot, three-component Strecker reaction of ketones with various amines using Brønsted acid catalyst.
Cyanation of aryl halides using palladium-complex as a catalyst.
| [Uses]
Trimethylsilyl Cyanide is used as a reagent in the synthesis of optically active cyanohydrins. Used in the preparation of Reissert compounds, which represent reactive polyamides. Toxic to humans. | [Preparation]
Trimethylsilyl cyanide, (CH3)3 Si-CN, is obtained by reacting trimethylsilyl chloride with an approximately equimolar amount of an alkali metal cyanide in the absence of water and in the presence of catalytic, sub-stoichiometric amounts of both an alkali metal iodide and N-methylpyrrolidone, at a temperature of from 15°-25° C.
Trimethylsilyl cyanide is very toxic. All reactions in this sequence should be carried out in a hood.
Preparation of trimethylsilyl cyanide
TRIMETHYLSILYL CYANIDE: CYANOSILATION OF p-BENZOQUINONE
| [Reactions]
Trimethylsilyl cyanide is a useful silicon reagent which reacts readily with aldehydes and ketones in the presence of catalytic amounts of Lewis acids or of cyanide ion, to give the trimethylsilyl ethers of the corresponding cyanohydrins (Evans,Carroll and Truesdale,1974).Even normally unreactive ketones react readily with trimethylsilyl cyanide due to the formation of the strong Si-O bond which displaces the equilibrium in favour of the derivative.The reaction provides a valuable alternative to the base-catalysed addition of hydrogen cyanide to carbonyl compounds which often gives only poor yields.Tetralone,for example,is reported not to form a cyanohydrin, but it gives a trimethylsilyl derivative in excellent yield.The silylated cyanohydrins can be hydrolysed to a-hydroxy acids(Corey, Crouse and Anderson,1975) and on reduction with lithium aluminium hydride they afford the corresponding 3-amino alcohols in excellent yield.This sequence provides a better route to these valuable intermediates (they are used in the ring expansion of cyclo- alkanones)than the classical methods through reaction of hydrogen cyanide or nitromethane with the carbonyl compound.The derivatives from aromatic aldehydes are excellent acyl anion equivalents and have been used in 'umpolung' conversion of aldehydes into ketones and acyloins by reaction of the derived anions with alkyl halides and aldehydes or ketones (Deuchert et al,1979;Hnig and Wehner,1979). | [Purification Methods]
The material should have only one sharp signal in the 1H NMR (in CCl4 with CHCl3 as internal standard) : 0.4ppm and IR with �max at 2210cm�1 [McBride & Beachall J Am Chem Soc 74 5247 1952, Prober J Am Chem Soc 77 3224 1955]; otherwise purify it by fractionating through an 18 x 1/4inch column. [Evers et al. J Am Chem Soc 81 4493 1959.] It has also been carefully distilled using a 60cm vacuum jacketed column. If the volume of sample is small, the cyanide can be chased (in the distillation) with xylene that had been previously distilled over P2O5. It is HIGHLY TOXIC and FLAMMABLE. [Evans et al. J Org Chem 39 914 1974, Beilstein 4 IV 3893.] |
|
|