| Identification | More | [Name]
Diphenoxylate | [CAS]
915-30-0 | [Synonyms]
DIPHENOXYLATE
1-(3-cyano-3,3-diphenylpropyl)-4-phenyl-4-piperidinecarboxylicacidethylester
1-(3-cyano-3,3-diphenylpropyl)-4-phenyl-4-piperidinecarboxylicaciethyl
1-(3-cyano-3,3-diphenylpropyl)-4-phenylisonipecoticacidethylester
1-(3-cyano-3,3-diphenylpropyl)-4-phenyl-isonipecoticacidethylester
1-(3-cyano-3,3-diphenylpropyl)-4-phenyl-isonipecoticaciethylester
2,2-diphenyl-4-(4-carbethoxy-4-phenylpiperidino)butyronitrile
ethyl1-(3-cyano-3,3-diphenylpropyl)-4-phenyl-4-piperidinecarboxylate
ethyl1-(3-cyano-3,3-diphenylpropyl)-4-phenylisonipecotate
ethyl 1-(3-cyano-3,3-diphenylpropyl)-4-phenylpiperidine-4-carboxylate
Diphenoxylate hydrochloride CP2000,BP98
DiphenoxyleteHCl
Diphenoxyletehydrochloride
4-Piperidinecarboxylic acid, 1-(3-cyano-3,3-diphenylpropyl)-4-phenyl-, ethyl ester
Isonipecotic acid, 1-(3-cyano-3,3-diphenylpropyl)-4-phenyl-, ethyl ester | [EINECS(EC#)]
213-020-1 | [Molecular Formula]
C30H32N2O2 | [MDL Number]
MFCD00072110 | [Molecular Weight]
452.59 | [MOL File]
915-30-0.mol |
| Hazard Information | Back Directory | [Originator]
Lomotil,Searle,US,1960 | [Uses]
Antiperistaltic. | [Uses]
This drug is a structural analog of meperidine and loperamide; however, it practically
duplicates all of the pharmacological properties of loperamide. Being analogous to lop�eramide, it is mainly used for treating diarrhea. Synonyms for this drug are fentanest, lep�rofen, and others. | [Definition]
ChEBI: A piperidinecarboxylate ester that is the ethyl ester of difenoxin. | [Indications]
Diphenoxylate (marketed in combination with atropine
as Lomotil in the United States) is chemically related
to both analgesic and anticholinergic compounds.
It is as effective in the treatment of diarrhea as the
opium derivatives, and at the doses usually employed, it
has a low incidence of central opioid actions. Diphenoxylate
is rapidly metabolized by ester hydrolysis to the
biologically active metabolite difenoxylic acid. | [Manufacturing Process]
A mixture of 23 parts of the ethyl ester of 4-phenylisonipecotic acid and 15
parts of 2,2-diphenyl-4-
omobutyronitrile in 19 parts of xylene is heated for
24 hours at 100-120°C and then cooled and filtered to remove the precipitate
of the hydro
omide of the ethyl ester of 4-phenylisonipecotic acid. The
filtrate is then extracted with dilute hydrochloric acid and the extract is
rendered alkaline by addition of concentrated aqueous potassium hydroxide
and extracted with ether. This ether extract is treated with gaseous hydrogen
chloride. The resulting precipitate is collected on a filter. The hydrochloride of
the ethyl ester of 2,2-diphenyl-4-(4'-carboxy-4'-phenyl-1'-
piperidino)butyronitrile thus obtained melts at about 220.5-222°C. See
meperidine hydrochloride for synthesis of 4-phenyl-isonipecotic acid ethyl
ester. | [Brand name]
Diarphem;Diarsed-neomycin;Diatro;Eldox;Logen;Lomanate;Lomax;Lomotil liquid;Lonox;Protector;Reasec;Saleton;Sedistal. | [Therapeutic Function]
Antidiarrheal | [World Health Organization (WHO)]
Diphenoxylate, a derivative of pethidine without analgesic
activity, is used in the symptomatic treatment of acute and chronic diarrhoea to
reduce intestinal motility. There is no clear evidence that it has any beneficial effect
in diminishing fluid losses and it has been associated with central nervous system
toxicity, particularly in children, which results in anorexia, nausea and vomiting,
headache, drowsiness, confusion, insomnia, dizziness, restlessness, euphoria and
depression. The World Health Organization recommends that diphenoxylate should
not be used for the management of diarrhoea in children and many countries have
since withdrawn products containing this compound indicated for paediatric use.
(Reference: (WHORUD) The Rational Use of Drugs, , , 1990) | [General Description]
Diphenoxylate is a weak opioid agonist and isavailable combined with atropine (Lomotil) for use as an antidiarrhealagent. At low doses, the opioid effect is minimal,and the atropine is added to dissuade abuse. One studyfound both codeine and loperamide to be superior to diphenoxylatefor treating chronic diarrhea.83 The manufacturerhas strict dosing guidelines for pediatric use because opioidintoxication and deaths from diphenoxylate have beenreported. | [Clinical Use]
Lomotil is recommended as adjunctive therapy in the management
of diarrhea. It is contraindicated in children under
2 years old and in patients with obstructive jaundice. | [Side effects]
Adverse reactions often caused by the atropine in the
preparation include anorexia, nausea, pruritus, dizziness,
and numbness of the extremities. | [Synthesis]
Diphenoxylate, ethyl ester of 1-(3-cyano-3,3-diphenylpropyl)-
4-phenylpiperidine-4-carboxylic acid (3.1.58), is also a drug of 4-phenylpiperidine series. In
practice there are two ways of making it. The first way is by the alkylation of the ethyl ester
of 4-phenylpiperidine-4-carboxylic acid (3.1.56) with 2,2-diphenyl-4-bromobutyronitrile,
which in turn is synthesized from 1-benzyl-4-phenyl-4-cyanopiperidine. The product under�goes ethanolysis in the presence of acid, followed by benzylation. The second way is a syn�thesis accomplished by alkylation of diphenylacetonitrile using ethyl ester of
1-(2-chloroethyl)-4-phenylpiperidine-4-carboxylic acid (3.1.57), which is synthesized by reaction of ethyl ester of 4-phenylpiperidine-4-carboxylic acid with |?-chloroethanol or eth�ylenoxide with the subsequent substitution of hydroxyl group, which results from the open�ing of the epoxide ring, by chlorine via action of thionyl chloride [37,38].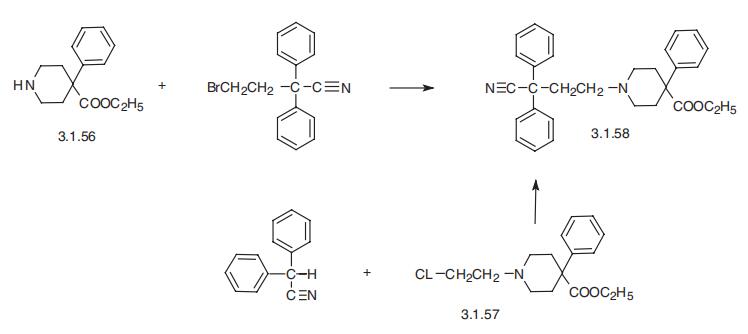
|
|