| Identification | Back Directory | [Name]
Guaifenesin | [CAS]
93-14-1 | [Synonyms]
GUAIPHENESIN
GUAIFENESINA
eguaifenesin
Guiaphenesin
Propanosedyl
Guaiphenesine
1 Guaifenesin
GUAIFENESINUM
Guaiaphenesin
Guaifenesin RS
Guaifenesin CRS
GUAIFENESIN USP
RAC GUAIFENESIN
Metfenossidiolo
| [EINECS(EC#)]
202-222-5 | [Molecular Formula]
C10H14O4 | [MDL Number]
MFCD00016873 | [MOL File]
93-14-1.mol | [Molecular Weight]
198.22 |
| Hazard Information | Back Directory | [Description]
Guaifenesin(93-14-1) is an oral expectorant drug. The expectorant action of guaifenesin is mediated by stimulation of the gastrointestinal tract. It is a common ingredient in prescription and over-the-counter medications used to treat cough due to colds and minor upper respiratory infections. Additionally, Guaifenesin is also a centrally-acting muscle relaxant and is used routinely in combination with analgesics and sedatives in large-animal veterinary surgery.
| [Chemical Properties]
This substance is a white crystalline powder with a melting point of 78.5-79°C and a boiling point of 215°C (2.53kPa). It is soluble in 20ml of water and ethanol, chloroform, glycerin, and dimethylformamide. It is easily soluble in benzene but insoluble in petroleum ether. It has a slightly bitter taste and a slightly particular odor. | [Originator]
GG Cen,Central,US,1975 | [History]
Guaifenesin was originally derived from the guaiac tree and used by Native Americans for health purposes. Synthesis of guaifenesin was first reported in 1912. Guaifenesin has been used in the treatment of respiratory diseases since the nineteenth century. The United States’ Food and Drug Administration (FDA) approved guaifenesin for use in overthe- counter medications in 1989. Guaifenesin is widely consumed alone and combined with antihistamines, cough suppressants, and decongestants. Guaifenesin is also a centrally acting muscle relaxant and is used routinely in combination with analgesics and sedatives in large-animal veterinary surgery. | [Uses]
Guaifenesin is a muscle relaxant with expectorant properties often used as an expectorant to facilitate the removal of phlegm from the airways in acute respiratory tract infections. Guaifenesin comes in tablet and capsule form, as syrup, as dissolving granules, and recently as an extended-release (longacting) tablet. The tablets, capsules, dissolving granules, and syrup are usually taken with or without food every 4 h as needed. The extended-release tablet is usually taken with or without food every 12 h.
| [Definition]
ChEBI: Guaifenesin is a member of methoxybenzenes. | [Manufacturing Process]
A mixture of o-methoxyphenol (57 g), glycidol (32 g) and pyridine (1 g) is
warmed to 95°C at which temperature a vigorous reaction takes place. The
reaction mixture is cooled to prevent the temperature rising above 110°C.
When the exothermic reaction has subsided the reactants are heated at 95°C
for one hour longer and then distilled under low pressure. The main fraction
boils in the range 176°C to 180°C/0.5 mm. It crystallizes on cooling.
Recrystallization from benzene gives the pure product, MP 78.5°C to 79.0°C. | [Brand name]
Mucinex (Adams). | [General Description]
Guaifenesin is an expectorant, widely used in the treatment of cough. Its mode of action involves the alleviating of cough discomfort by increasing sputum volume and decreasing its viscosity, thus resulting in effective cough.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. | [Mechanism of action]
Guaiphenesin facilitates secretion from bronchial mucous membranes, thus relieving a
cough in colds, bronchitis, and bronchial asthma. | [Synthesis]
Guaiphenesin, 3-(o-methoxyphenoxy)-1,2-propandiol (23.2.3), is synthesized
by reacting guiacol with 3-chloropropan-1,2-diol or with glycidol.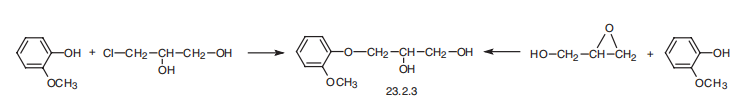
| [Veterinary Drugs and Treatments]
In veterinary medicine, guaifenesin is used to induce muscle relaxation
and restraint
as an adjunct to anesthesia for short procedures
(30 – 60 minutes) in large and small animal species. There are combination
oral products containing guaifenesin for treating respiratory
conditions in horses.
In human medicine, guaifenesin has long been touted as an oral
expectorant, but definitive proof of its efficacy is lacking. | [Environmental Fate]
Guaifenesin’s production and use as veterinary and human
medicines may result in its release to the environment through
various waste streams.
If released to air, an estimated vapor pressure of
1.5× 10–6 mm Hg at 25 ℃ indicates that guaifenesin will
exist in both the vapor and particulate phase.
Based upon an estimated Henry’s law constant of
4.4 × 10-11 atm-m3 mol-1, volatilization from moist soil
surfaces or from water surfaces are not expected to be important
fate processes for guaifenesin.
Guaifenesin is expected to have high mobility in soil based
upon an organic carbon–water partition coefficient (Koc) of
140, which indicates that it will have more solubility in water
and is less likely to adsorb onto organic matter in soil and
plants. | [Toxicity evaluation]
Guaifenesin or 93-14-1 is an adrenergic antagonist in a class of medications
called expectorants. It stimulates afferent receptors in the
gastric mucosa, reflexively increasing glandular secretion by the
respiratory epithelium promoting lower respiratory tract
drainage by thinning bronchial secretions, lubricating irritated
respiratory tract membranes through increased mucous flow,
and facilitating removal of viscous mucus. The onset of action
appears to be within 15–30 min. Guaifenesin is believed to
alleviate cough discomfort by improving sinus and bronchial
drainage, increasing sputum volume, and decreasing sputum
viscosity, thereby promoting effective cough. In one study, the
effect of guaifenesin to increase mucociliary clearance from the
lung was greater in patients with chronic bronchitis than in
healthy subjects.
In another study, guaifenesin inhibited the cough reflex
sensitivity in subjects with an upper respiratory tract infection
(cough receptors are transiently hypersensitive), but not in
healthy volunteers. Possible mechanisms include a central
antitussive effect or a peripheral effect by increased sputum
volume serving as a physical barrier, shielding cough receptors
within the respiratory epithelium.
As a centrally acting muscle relaxant, guaifenesin is believed
to depress or block nerve impulse transmission at the internuncial
neuron level of the subcortical areas of the brain, brain
stem, and spinal cord. It also has mild analgesic and sedative
actions.
| [References]
[1] Peter V. Dicpinigaitis and Yvonne E. Gayle, Effect of Guaifenesin on Cough Reflex Sensitivity, 2003, vol. 124, 2178-2181 DOI:10.1378/CHEST.124.6.2178
[2] Leonid Kagan, Eran Lavy and Ammon Hoffmann, Effect of mode of administration on guaifenesin pharmacokinetics and expectorant action in the rat model, Pulmonary Pharmacology & Therapeutics, 2009, vol. 22, 260-265 DOI:10.1016/j.pupt.2008.12.020
[3] Sittig's Pharmaceutical Manufacturing Encyclopedia
[4] Synthesis of Essential Drugs (2006, Elsevier)
[5] Plumb's Veterinary Drug Handbook
[6] https://pubchem.ncbi.nlm.nih.gov/compound/Guaifenesin |
|
|