Acetophenon Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
FARBLOSE FLüSSIGKEIT ODER WEISSE KRISTALLE MIT CHARAKTERISTISCHEM GERUCH.
ARBEITSPLATZGRENZWERTE
TLV: 10 ppm (als TWA); (ACGIH 2005).
MAK nicht festgelegt (DFG 2005).
AUFNAHMEWEGE
Aufnahme in den Körper durch Inhalation, über die Haut und durch Verschlucken.
INHALATIONSGEFAHREN
Beim Verdampfen bei 20°C tritt langsam eine gesundheitsschädliche Kontamination der Luft ein; viel schneller jedoch beim Versprühen oder Dispergieren.
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION: Die Substanz reizt die Augen. Möglich sind Auswirkungen auf das Zentralnervensystem. Exposition gegenüber hohen Konzentrationen kann zu Bewusstlosigkeit führen.
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Die Flüssigkeit entfettet die Haut.
LECKAGE
Ausgelaufene Flüssigkeit möglichst in abdichtbaren Behältern sammeln. Reste mit Sand oder inertem Absorptionsmittel aufnehmen und an einen sicheren Ort bringen. Persönliche Schutzausrüstung: Atemschutzgerät, A/P2-Filter für organische Dämpfe und schädlichen Staub.
R-Sätze Betriebsanweisung:
R22:Gesundheitsschädlich beim Verschlucken.
R36:Reizt die Augen.
R63:Kann das Kind im Mutterleib möglicherweise schädigen.
R43:Sensibilisierung durch Hautkontakt möglich.
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
R23/24/25:Giftig beim Einatmen, Verschlucken und Berührung mit der Haut.
R45:Kann Krebs erzeugen.
R39/23/24/25:Giftig: ernste Gefahr irreversiblen Schadens durch Einatmen, Berührung mit der Haut und durch Verschlucken.
R11:Leichtentzündlich.
S-Sätze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36/37:Bei der Arbeit geeignete Schutzhandschuhe und Schutzkleidung tragen.
S24/25:Berührung mit den Augen und der Haut vermeiden.
S23:Gas/Rauch/Dampf/Aerosol nicht einatmen(geeignete Bezeichnung(en) vom Hersteller anzugeben).
S53:Exposition vermeiden - vor Gebrauch besondere Anweisungen einholen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn möglich, dieses Etikett vorzeigen).
S16:Von Zündquellen fernhalten - Nicht rauchen.
S7:Behälter dicht geschlossen halten.
Aussehen Eigenschaften
C8H8O; Methylphenylketon, Acetylbenzol. Farblose Flüssigkeit mit charakteristischem Geruch.
Gefahren für Mensch und Umwelt
Gesundheitsschädlich beim Verschlucken. Reizt die Augen. Resorption führt zur Narkose.
Nicht mit starken Oxidationsmitteln, Laugen und Säuren in Berührung bringen.
LD
50 (oral, Ratte): 815 mg/kg
Schutzmaßnahmen und Verhaltensregeln
Geeignete Schutzhandschuhe als kurzzeitiger Spritzschutz.
Verhalten im Gefahrfall
Mit flüssigkeitsbindendem Material, z.B. Rench Rapid aufnehmen. Der Entsorgung zuführen. Nachreinigen.
Kohlendioxid, Pulver, Schaum.
Brennbar. Im Brandfall kann Kohlenmonoxid entstehen.
Erste Hilfe
Nach Hautkontakt: Mit reichlich Wasser abspülen.
Nach Augenkontakt: Mit reichlich Wasser bei geöffnetem Lidspalt mindestens 10 Minuten ausspülen. Sofort Augenarzt hinzuziehen.
Nach Einatmen: Frischluft.
Nach Verschlucken: Reichlich Wasser trinken lassen. Erbrechen auslösen. Arzt hinzuziehen.
Nach Kleidungskontakt: Kontaminierte Kleidung entfernen.
Ersthelfer: siehe gesonderten Anschlag
Sachgerechte Entsorgung
Als halogenfreie, organische Lösemittelabfälle.
Beschreibung
Acetophenone is the simplest aromatic ketone and is a clear liquid/crystal and very slightly
soluble in water with a sweet pungent taste and odour resembling oranges. It is used as
a polymerisation catalyst for the manufacture of olefins. Acetophenone is used in perfumery
as a fragrance ingredient in soaps, detergents, creams, lotions, and perfumes; as a
flavouring agent in foods, non-alcoholic beverages, and tobacco; as a specialty solvent for plastics and resins; as a catalyst for the polymerisation of olefins; and as a photosensitiser
in organic syntheses. Acetophenone is a raw material for the synthesis of some pharmaceuticals
and is also listed as an approved excipient by the U.S. FDA. Acetophenone occurs
naturally in many foods such as apple, apricot, banana, and beef. Acetophenone has been detected in ambient
air and drinking water; exposure of the general public may occur through the inhalation
of contaminated air or the consumption of contaminated water. It is highly flammable
and will get easily ignited by heat, sparks, or flames, and the vapours may form explosive
mixtures with air.
Chemische Eigenschaften
Acetophenone is a colorless, oily liquid with a sweet, floral odor.It is a naturally occurring component of a large number of foods and essential oils.
Acetophenone can be hydrogenated catalytically to 1-phenylethanol. It is obtained as a by-product in the Hock phenol synthesis and is purified from the high-boiling residue by distillation. The quantities obtained from this source satisfy the present demand.
Acetophenone is used for perfuming detergents and industrial products and is an intermediate in the synthesis of other fragrance materials.
Occurrence
Reported found in cocoa, beef, raspberry, peas, and concord grape
Verwenden
Acetophenone is a reagent used in the production of fragrances and resin polymers.
Definition
ChEBI: Acetophenone is a methyl ketone that is acetone in which one of the methyl groups has been replaced by a phenyl group. It has a role as a photosensitizing agent, an animal metabolite and a xenobiotic.
Vorbereitung Methode
Most methyl phenyl ketone originates from the Hock process for the production of phenol from isopropylbenzene (→Phenol); it is isolated from the residue of this process. In addition, acetophenone can be obtained as a main product by selective decomposition of cumene hydroperoxide in the presence of copper catalysts at 100℃:
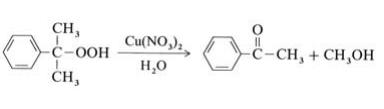
A second possibility is the oxidation of ethylbenzene with air or oxygen at 130℃ and 0.5 MPa. Catalysts used include cobalt salts or manganese salts of naphthenic or fatty acids. Conversion of ethylbenzene is limited to ca. 25 % to minimize the byproducts 1-phenylethanol and benzoic acid. A third method is the Friedel – Crafts acetylation of benzene with acetic anhydride, but this is not of industrial importance.
synthetische
From benzene and acetylchloride in the presence of aluminum chloride or by catalytic oxidation of ethyl benzene; also
prepared by fractional distillation and crystallization from the essential oil of Stirlingia latifolia.
Allgemeine Beschreibung
Acetophenone appears as a colorless liquid with a sweet pungent taste and odor resembling the odor of oranges. Freezes under cool conditions. Slightly soluble in water and denser than water. Hence sinks in water. Vapor heavier than air. A mild irritant to skin and eyes. Vapors can be narcotic in high concentrations. Used as a flavoring, solvent, and polymerization catalyst.
Air & Water Reaktionen
Slightly soluble in water.
Reaktivität anzeigen
Acetophenone reacts with many acids and bases liberating heat and flammable gases (e.g., H2). Reacts with many oxidizing agents. Reacts with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. The amount of heat in these reactions may be sufficient to start a fire in the unreacted portion. Incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides.
Health Hazard
Acetophenone is an irritant, mutagen, and amildly toxic compound. In rabbits 0.77 mgproduced severe eye irritation, but the actionon skin was mild. In mice, subcutaneousadministration of this compound producedsleep; a dose of 330 mg/kg was lethal.
LD50 value, intraperitoneal (mice): 200mg/kg
No symptoms of severe toxicity, nor its carcinogenicityin humans, has been reported..
Brandgefahr
Combustible liquid; flash point (closed cup)
82°C (180°F); vapor pressure 1 torr at
37°C (98.6°F); vapor density 4.1 (air = 1);
autoignition temperature 570°C (1058°F);
fire-extinguishing agent: dry chemical, foam,
or CO2; water may cause frothing, but it can
be used to flush and dilute the spill. Its reaction
with strong oxidizers may be violent.
Sicherheitsprofil
Poison by intraperitoneal and subcutaneous routesModerately toxic by ingestion. A skin and severe eye irritant. Mutation data reported. Narcotic in high concentration. A hypnotic. Flammable liquid. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and fumes. See also IGTONES
mögliche Exposition
Acetophenone is used as a solvent and in perfume manufacture to impact a pleasant jasmine or orange-blossom odor. It is used as a catalyst in olefin polymerization and as a flavorant in tobacco. It is also used in the synthesis of pharmaceuticals
Carcinogenicity
No carcinogenicity studies were
identified for acetophenone. The U.S. EPA has classified
acetophenone as a Category D, not classifiable as to human
carcinogenicity.
Environmental Fate
It is unclear what mechanism is responsible for the central
nervous system depression observed following high doses of
acetophenone. In vitro evaluations have demonstrated that
acetophenone suppresses voltage-gated ion channels in olfactory
receptor cells and retinal neurons; however, it is unclear if
this is related to any of the observed toxicity in animal studies.
Stoffwechsel
At one time, acetophenone was used as a hypnotic. Its conversion to benzoic acid and methylphenylcarbinol in dogs and rabbits was observed by a number of early workers. Small amounts are also excreted as mandelic acid. In the rabbit about half the dose is excreted as methylphenylcarbinyl glucuronide and about 20 % as hippuric acid. It is probable that the ketone is first asymmetrically reduced to the carbinol, which is the precursor of benzoic and mandelic acids.
Versand/Shipping
UN1993 Flammable liquids, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, Technical Name Required.
läuterung methode
Dry it by fractional distillation or by standing with anhydrous CaSO4 or CaCl2 for several days, followed by fractional distillation under reduced pressure (from P2O5, optional), and careful, slow and repeated partial crystallisations from the liquid at 0o excluding light and moisture. It can also be crystallised at low temperatures from isopentane. Distillation can be followed by purification using gas-liquid chromatography [Earls & Jones J Chem Soc, Faraday Trans 1 71 2186 1975.] [Beilstein 7 H 271, 7 IV 619.] § A commercial polystyrene supported version is available — scavenger resin (for diol substrates).
Inkompatibilitäten
May form explosive mixture with air. See flash point, above. Reacts violently with strong oxidizers, many acids, bases, amines, amides, and inorganic hydroxides; alkali metals; hydrides, and nitrides. Reacts with reducing agents; alkali metals; hydrides, nitrides. Contact with all preceding materials release heat and flammable gases, including hydrogen; the heat may be sufficient enough to result in fire. Incompatible with aldehydes, aliphatic amines, alkanolamines, cyanides, isocyanates, organic acids, peroxides; perchloric acid. May attack plastics, and some rubbers and coatings
Waste disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration, preferably with a flammable solvent
Acetophenon Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
polyquinoxaline
3-Phenyl-1H-pyrazole-5-carboxamide ,97%
METHYL 2-AMINO-4-PHENYLTHIOPHENE-3-CARBOXYLATE
Calcium-(2R-cis)-hydrogen(3-methyloxiranyl)phosphonat
Miconazolnitrat
Hydratropaldehyd
2-AMINO-4-PHENYL-THIOPHENE-3-CARBOXYLIC ACID ETHYL ESTER
FLUOXETINE HYDROCHLORIDE
(-)-Bis[(S)-1-phenylethyl]amine
(-)-BIS[(S)-1-PHENYLETHYL]AMINE HYDROCHLORIDE
4-[3-[4-(1,1-Dimethylethyl)phenyl]-2-methylpropyl]-2,6-dimethylmorpholin
2,2-DICHLOROACETOPHENONE
2,5-Diphenyloxazol
alpha-Methylbenzolmethanol
DL-α-Methylbenzylamin
Alkofanone
(R,R)-(+)-BIS(ALPHA-METHYLBENZYL)AMINE HYDROCHLORIDE
Naftifine
4,4,4-Trifluor-1-phenylbutan-1,3-dion
3-Amino-5-phenylpyrazole
Acebutolol
3-PHENYL-1H-PYRAZOLE-5-CARBOHYDRAZIDE
Azelastine
1-Methyl-2-phenylindol
Enrofloxacin hydrochloride
1-Methyl-2-phenyl-1H-indol-3-carbaldehyd
METHYL ALPHA-BROMOPHENYLACETATE
1-Phenylbutan-1,3-dion
1-Phenylethylamin (1)
Fosfomycin
5-Phenyl-1H-pyrazole-3-carboxylic acid
A-METHYL-3-PHENOXYBENZENEACETALDEHYDE
3,5-Diphenyl-1H-pyrazol
Enrofloxacin
5-PHENYL-2H-PYRAZOLE-3-CARBOXYLIC ACID ETHYL ESTER
2-(2-Iminothiazolidin-3-yl)-1-phenylethan-1-onmonohydrobromid
Ethyl-2,3-epoxy-3-phenylbutyrat
5-PHENYL-3H-THIENO[2,3-D]PYRIMIDIN-4-ONE
Econazol
Oxyfedrine

