Oxazolidinone
- CAS No.
- 51667-26-6
- Chemical Name:
- Oxazolidinone
- Synonyms
- Oxazolidinone;(R)-(-)-4-ISOPROPYL-3-PROPIMYL-2-OXAZOLIDINOE
- CBNumber:
- CB01028254
- Molecular Formula:
- C3H5NO2
- Molecular Weight:
- 87.0773
- MDL Number:
- MOL File:
- Mol file
| FDA UNII | Z4D49W92PP |
|---|
Oxazolidinone Chemical Properties,Uses,Production
Preparation
The 1,2-amino alcohol (10.0 mmol), solvent (8.0 mL), and n-Bu2SnO (1.0 mmol) were placed in a 50 mL autoclave. CO2 was then introduced at an initial pressure of 5 MPa, and the autoclave was heated at 180 ℃ for 16 h. The reaction solution obtained was then analyzed by GLC. The products were isolated by fractional distillation of the reaction solution under reduced pressure. When necessary, i.e. for characterization, they could be further purified by preparative GLC. The products were identified by comparing their FT-IR-, mass-, and 1H NMR spectra with those of corresponding authentic samples.
Definition
ChEBI: Oxazolidin-2-one is an oxazolidinone that is 1,3-oxazolidine with an oxo substituent at position 2. It has a role as a metabolite. It is an oxazolidinone and a carbamate ester.
Preparation
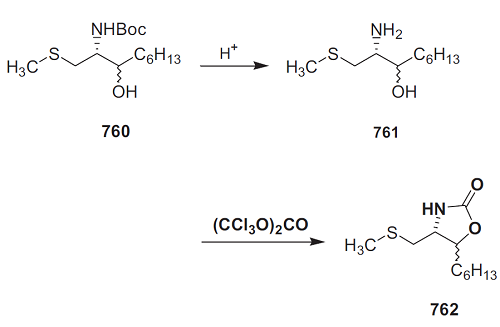
The amino alcohol 761 (1 equiv.) was dissolved in dichloromethane (10 mL/mmol) and triphosgene (0.3 equiv.) and triethylamine (1 equiv.) were added. After 1 h, the reaction mixture was hydrolyzed with H2O. The organic layer was separated, washed successively with saturated aq. NaHCO3 solution and saturated brine, dried over Mg2SO4, and concentrated in vacuo. The residue was chromatographed on silica gel (n-hexane/ethyl acetate, 1:1); threo isomer 762: 54.6 mg (67%) from 85 mg (0.35 mmol) of threo-761 as a pale oil; erythro isomer 762: 124 mg (91%) from 100 mg (0.26 mmol) of erythro-761.
Pharmaceutical Applications
The oxazolidinones are a novel class of synthetic antimicrobial agents unrelated to any other antibacterial drug class. They were originally developed as monoamine oxidase inhibitors for treatment of depression, with subsequent recognition of their antimicrobial properties. The first members of the group to emerge exhibited potent activity against Gram-positive organisms, but were not developed for human use owing to toxicity in animal models. In the 1990s two less toxic oxazolidinones were developed: eperezolid, a piperazine derivative, and linezolid, a morpholine derivative. Linezolid exhibited the more favorable pharmacokinetic profile and was subsequently licensed for human use.
Numerous chemical analogs have been screened in a search for compounds with enhanced potency, a broader spectrum of activity or a more favorable side effect profile. Several promising candidates have been described, but linezolid remains the only oxazolidinone currently available.
These drugs are characterized by activity against Gram-positive organisms including methicillin-resistant Staphylococcus aureus, penicillin-resistant pneumococci and vancomycin-resistant enterococci. They are inactive against most Gram-negative species, although some investigational compounds are active against fastidious Gramnegative bacteria including Haemophilus influenzae. They have potentially useful activity against mycobacteria, including multiresistant isolates of Mycobacterium tuberculosis. Resistance among Grampositive organisms remains very uncommon (<0.5%) among clinical isolates.
Oxazolidinone Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Dvr Chemicals Private Limited | -- | India | 10 | 58 | |
| XIANG DING CHEMICAL INTL CO.,LTD | -- | China | 4372 | 63 | |
| Shanghai International Pharmaceutical Co., Ltd | -- | ronghua@wzpharm.com | China | 117 | 51 |
| ecochem international chemical broker | -- | export@ecochem.dk | Europe | 6385 | 66 |
| kemikalieimport | -- | Sales@kemikalieimport.dk | Europe | 6699 | 47 |





