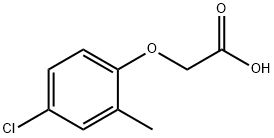
2-Methyl-4-chlorophenoxyacetic acid
- CAS No.
- 94-74-6
- Chemical Name:
- 2-Methyl-4-chlorophenoxyacetic acid
- Synonyms
- MCP;MCPA;MCPA Ester;2-Methyl-4-chlorophenoxyacetic;(4-CHLORO-2-METHYLPHENOXY)ACETIC ACID;2-(4-CHLORO-2-METHYLPHENOXY)ACETIC ACID;Mecpa;EMPAL;MCCPA;2M2Kh
- CBNumber:
- CB0165198
- Molecular Formula:
- C9H9ClO3
- Molecular Weight:
- 200.62
- MDL Number:
- MFCD00004306
- MOL File:
- 94-74-6.mol
- MSDS File:
- SDS
| Melting point | 114-118 °C (lit.) |
|---|---|
| Boiling point | 288.02°C (rough estimate) |
| Density | 1.2799 (rough estimate) |
| refractive index | 1.5230 (estimate) |
| Flash point | 2 °C |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | water: insoluble(lit.) |
| form | Solid |
| pka | 3.14±0.10(Predicted) |
| color | White to Light yellow to Light orange |
| Water Solubility | 1.174g/L(25 ºC) |
| Merck | 14,5764 |
| BRN | 2051752 |
| CAS DataBase Reference | 94-74-6(CAS DataBase Reference) |
| EWG's Food Scores | 2-3 |
| FDA UNII | D888C394VO |
| NIST Chemistry Reference | [(4-Chloro-o-tolyl)oxy]acetic acid(94-74-6) |
| Pesticides Freedom of Information Act (FOIA) | MCPA |
| EPA Substance Registry System | MCPA (94-74-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H315-H318-H410 | |||||||||
| Precautionary statements | P264-P273-P280-P301+P312-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,F,N | |||||||||
| Risk Statements | 22-38-41-36-20/21/22-11-50/53 | |||||||||
| Safety Statements | 26-37-39-36-16-61-60-36/37 | |||||||||
| RIDADR | UN 2765 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | AG1575000 | |||||||||
| HazardClass | 9 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29189900 | |||||||||
| Toxicity | LD50 orally in rats: 700 mg/kg (Rowe, Hymas) | |||||||||
| NFPA 704 |
|
2-Methyl-4-chlorophenoxyacetic acid price More Price(16)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 45555 | MCPA PESTANAL | 94-74-6 | 250mg | $41.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 25190 | 4-Chloro-2-methylphenoxyacetic acid technical, ≥95.0% (T) | 94-74-6 | 100g | $143.4 | 2024-03-01 | Buy |
| TCI Chemical | C0206 | (4-Chloro-2-methylphenoxy)acetic Acid >98.0%(T) | 94-74-6 | 25g | $50 | 2024-03-01 | Buy |
| TCI Chemical | C0206 | (4-Chloro-2-methylphenoxy)acetic Acid >98.0%(T) | 94-74-6 | 500g | $385 | 2024-03-01 | Buy |
| TRC | C369470 | 4-Chloro-2-methylphenoxyacetic acid | 94-74-6 | 250mg | $85 | 2021-12-16 | Buy |
2-Methyl-4-chlorophenoxyacetic acid Chemical Properties,Uses,Production
Description
MCPA is a colorless crystalline solid. Molecular weight= 200.63; Freezing/Melting point=118.8℃; Vapor pressure= 1.5×106 mmHg at 20℃. Insoluble in water.
Chemical Properties
MCPA is a colorless crystalline solid
Chemical Properties
White, crystalline solid. Free acid insoluble in water but sodium and amine salts are soluble.
Uses
(4-Chloro-2-methylphenoxy)acetic Acid is a herbicide.Environmental toxin on US EPA Toxic Release Inventory list (TRI) list.
Uses
Systemic postemergence herbicide used to control annual and perennial weeds in cereals, rice, flax, vines, peas, potatoes, asparagus, grassland and turf.
Uses
Labelled MCPA (C369470). Herbicide.
Definition
ChEBI: A chlorophenoxyacetic acid that is (4-chlorophenoxy)acetic acid substituted by a methyl group at position 2.
General Description
Colorless plates. Corrosive. Practically insoluble in water. Used as an herbicide.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
2-Methyl-4-chlorophenoxyacetic acid is a chlorinated benzoic acid derivative. Reacts as a weak acid to neutralize bases, both organic (for example, the amines) and inorganic. May corrode iron, steel, and aluminum parts and containers if moist. Reacts with cyanide salts in the presence of moisture to generate gaseous hydrogen cyanide. May react if moist with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A variety of products is possible. Like other acids, may initiate polymerization reactions or catalyze other reactions. is a chlorinated carboxylic acid herbicide. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in 2-Methyl-4-chlorophenoxyacetic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions.
Agricultural Uses
Herbicide: A U.S. EPA restricted Use Pesticide (RUP) as MCPA, sodium salt. MCPA is a systemic post-emergence phenoxy herbicide used to control broadleaf annual and perennial weeds (including thistle and dock) in cereals, flax, rice, vines, peas, potatoes, grasslands, forestry applications, and on rights-of-way. It is very compatible with many other compounds and may be used in formulation with many other products, including bentazone, bromoxynil, 2,4-D, dicamba, fenoxaprop, MCPB, mecoprop, thifensulfuron, and tribenuron.
Trade name
ACME MCPA AMINE 4®; AGRITOX®; AGROXONE®; AGROZONE®; AGSCO®; ANICON KOMBI®; ANICON M®; BANLENE®; BLESEL MC®; BORDERMASTER®; BROMINAL M & PLUS®; CAMBILENE®; CHEYENNE®; CHIMAC OXY®; CHIPTOX®; CHWASTOX®; CORNOX M®; DAKOTA®; DED WEED®; DICOPUR-M®; DICOTEX®; DOW MCP AMINE WEED KILLER®; DYVEL®; EH1356 HERBICIDE®; EMCEPAN®; EMPAL®; ENVOY®; HEDAPUR M 52®; HEDAREX M®; HEDONAL M®; HERBICIDE M®; HORMOTUHO®; HORNOTUHO®; KILSEM®; 4 K-2 M®; KVK®; LEGUMEX DB®; LEUNA M®; LEYSPRAY®; LINORMONE®; M 40®; 2 M-4C®; 2 M-4KH®; MALERBANE®; MAYCLENE®; MEPHANAC®; MIDOX®; MXL®; OKULTIN®; PHENOXYLENE 50®; PHENOXYLENE PLUS®; PHENOXYLENE SUPER®; RAZOL DOCK KILLER®; RHOMENE®; RHONOX®; SHAMOX®; B-SELEKTONON M®; SEPPIC MMD®; TILLER®; TRIMEC®; U 46®; VACATE®; VESAKONTUHO®; WEEDAR®; WEEDAR MCPA CONCENTRATE®; WEEDONE MCPA ESTER®; WEED RHAP®; ZELAN®
Safety Profile
Suspected carcinogen. Poison by subcutaneous and intravenous routes. Moderately toxic by ingestion. Human systemic effects by ingestion: blood pressure decrease and coma. Experimental teratogenic and reproductive effects. Mutation data reported. An herbicide. When heated to decomposition it emits toxic fumes of Cl-.
Potential Exposure
A potential danger to those involved in the manufacture, formulation, and application of this postemergence herbicide, used for control of broadleaf weeds in agricultural applications.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least 15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Environmental Fate
Biological. Cell-free extracts isolated from Pseudomonas sp. in a basal salt medium
degraded MCPA to 4-chloro-o-cresol and glyoxylic acid (Gamar and Gaunt, 1971).
Soil. Residual activity in soil is limited to approximately 3–4 months (Hartley and
Kidd, 1987).
Plant. The penetration, translocation and metabolism of radiolabeled MCPA in a
cornland weed (Galium aparine) was studied by Leafe (1962). Carbon dioxide was identified
as a metabolite but this could only account 7% of the applied MCPA. Though no
Photolytic. When MCPA in dilute aqueous solution was exposed to summer sunlight
or an indoor photoreactor (l >290 nm), 2-methyl-4-chlorophenol formed as the major
product as well as o-cresol and 4-chloro-2-formylphenol (Soderquist and Crosby, 1975).
Clapés et al. (1986) studied the photodecomposition of aqueous solution of MCPA (120
ppm, pH 5.4, 25°C) in a photoreactor equipped with a high pressure mercury lamp. After three minutes of irradiation, 4-chloro-2-methylphenol formed as an intermediate which
degraded to 2-methylphenol. Both compounds were not detected after 6 minutes of irradiation;
however, 1,4-dihydroxy-2-methylbenzene and 2-methyl-2,5-cyclohexadiene-1,4-
dione formed as major and minor photodecomposition products, respectively. The same
experiment was conducted using simulated sunlight (l <300 nm) in the presence of
riboflavin, a known photosensitizer. 4-Chloro-2-methylphenol and 4-chloro-2-methylbenzyl
formate formed as major and minor photoproducts, respectively (Clapés et al., 1986).
Ozone degraded MCPA in dilute aqueous solution with and without UV light (l >300
nm) (Benoit-Guyod et al., 1986).
Chemical/Physical. Reacts with alkalies forming water soluble salts (Hartley and Kidd,
1987). Ozonolysis of MCPA in the dark yielded the following benzenoid intermediates:
4-chloro-2-methylphenol, its formate ester, 5-chlorosalicyaldehyde, 5-chlo
storage
Color Code—Blue: Health Hazard/Poison: Store in a secure poison location. Prior to working with this chemical you should be trained on its proper handling and storage. Store in tightly closed containers in a cool, wellventilated area. A regulated, marked area should be established where this chemical is handled, used, or stored in compliance with OSHA Standard 1910.1045.
Shipping
UN3345 Phenoxyacetic acid derivative pesticide, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required
Purification Methods
It is insoluble in H2O (solubility is 0.55g/L at 20o) and recrystallises from *C6H6 or chlorobenzene as plates [J.nsson et al. Acta Chem Scand 6 993 1952]. The S-benzylisothiouronium salt has m 164-165o, and the Cu2+ salt has m 247-249o(dec) [Armarego et al. Nature 183 1176 1959, UV: Duvaux & Grabe Acta Chem Scand 4 806 1950, IR: J.berg Acta Chem Scand 4 798 1950]. [Beilstein 6 IV 1991.] It is a plant growth substance and a herbicide.
Incompatibilities
A weak acid. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic, and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur). Incompatible with alkalis.
Waste Disposal
Incineration with added flammable solvent; incinerator equipped with fume scrubber. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office
2-Methyl-4-chlorophenoxyacetic acid Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12452 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 | kyra@quanjinci.com | China | 1532 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15371 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Klong Industrial Co., Ltd | 0086-519-68231162 | info@klongchem.com | CHINA | 131 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
View Lastest Price from 2-Methyl-4-chlorophenoxyacetic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-11-29 | 2-Methyl-4-chlorophenoxyacetic acid
94-74-6
|
US $30.00 / kg | 1kg | 99% | 5000000 tons | Wuhan Quanjinci New Material Co.,Ltd. | |
 |
2023-08-18 | 2-Methyl-4-chlorophenoxyacetic acid
94-74-6
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2022-11-01 | 2-Methyl-4-chlorophenoxyacetic acid
94-74-6
|
US $0.00-0.00 / KG | 1KG | 99% | 20 mt | Hebei Guanlang Biotechnology Co., Ltd. |
-

- 2-Methyl-4-chlorophenoxyacetic acid
94-74-6
- US $30.00 / kg
- 99%
- Wuhan Quanjinci New Material Co.,Ltd.
-

- 2-Methyl-4-chlorophenoxyacetic acid
94-74-6
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- 2-Methyl-4-chlorophenoxyacetic acid
94-74-6
- US $0.00-0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.