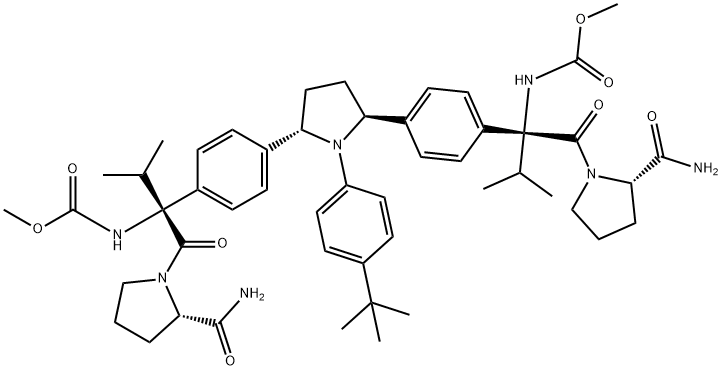
ABT-267
- CAS No.
- 1258226-87-7
- Chemical Name:
- ABT-267
- Synonyms
- Ontave;ABT-267;ombitasvir;Mr. He wei;Viekira Pak);ABT-267/Ombitasvir;Ombitasvir(ABT-267);Ombitasvir, Paritaprevir, and Ritonavir;2,2'-[[(2S,5S)-1-[4-(1,1-Dimethylethyl)phenyl]-2,5-pyrrolidinediyl]di-4,1-phenylene]bis[N-(methoxycarbonyl)-L-valyl-L-prolinamide;L-Prolinamide, 2,2'-[[(2S,5S)-1-[4-(1,1-dimethylethyl)phenyl]-2,5-pyrrolidinediyl]di-4,1-phenylene]bis[N-(methoxycarbonyl)-L-valyl-
- CBNumber:
- CB02716109
- Molecular Formula:
- C50H67N7O8
- Molecular Weight:
- 894.11
- MDL Number:
- MFCD28386270
- MOL File:
- 1258226-87-7.mol
- MSDS File:
- SDS
| Melting point | >177°C (dec.) |
|---|---|
| Boiling point | 1065.6±65.0 °C(Predicted) |
| Density | 1.223±0.06 g/cm3(Predicted) |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 10.92±0.46(Predicted) |
| form | Solid |
| color | Off-White to Pale Beige |
| FDA UNII | 2302768XJ8 |
| Protein binding | Ombitasvir: 99.9; Paritaprevir: 97-98.6; Ritonavir: 98-99% |
|---|---|
| Excreted unchanged in urine | Ombitasvir: 0.03; Paritaprevir: 0.05; Ritonavir: 3.5% |
| Volume of distribution | Ombitasvir: 173 Litres; Paritaprevir: 103 Litres; Ritonavir: 0.4(L/kg) |
| Biological half-life | Ombitasvir: 21-25; Paritaprevir: 5.5; Ritonavir: 3-5 / Unchanged |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319 |
| Precautionary statements | P264-P280-P302+P352-P337+P313-P305+P351+P338-P362+P364-P332+P313 |
ABT-267 price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 24116 | Ombitasvir ≥98% | 1258226-87-7 | 500μg | $106 | 2024-03-01 | Buy |
| Cayman Chemical | 24116 | Ombitasvir ≥98% | 1258226-87-7 | 1mg | $198 | 2024-03-01 | Buy |
| Cayman Chemical | 24116 | Ombitasvir ≥98% | 1258226-87-7 | 5mg | $672 | 2024-03-01 | Buy |
| TRC | O633200 | Ombitasvir | 1258226-87-7 | 25mg | $1390 | 2021-12-16 | Buy |
| ChemScene | CS-5330 | Ombitasvir 99.79% | 1258226-87-7 | 100mg | $940 | 2021-12-16 | Buy |
ABT-267 Chemical Properties,Uses,Production
Description
Ombitasvir hydrate is a NS5A non-nucleoside polymerase inhibitor which is approved as part of a four drug combination for the treatment of adults with genotype 1 hepatitis C virus infection including those with compensated cirrhosis. The four drug combination treatment consists of ombitasvir, paritaprevir (XXVII), ritonavir, and dasabuvir (X). This combination treatment is marketed as Viekira Pak and was developed by Abbvie as an all oral treatment that eliminates the need for pegylated interferon- a injections.
Uses
Ombitasvir is a pharmaceutical drug that is used in the treatment of hepatitis C virus in patients with HCV genotype 1 infection. It inhibits an important viral phosphoprotein, NS5A, which is involved in viral replication, assembly, and secretion.
Definition
ChEBI: A dipeptide derivative which is used which is in combination with dasabuvir sodium hydrate, paritaprevir and ritonavir (under the trade name Viekira Pak) for treatment of chronic hepatitis C virus genotype 1 infection as well as cirrhosis of the liver.
Clinical Use
Treatment of chronic hepatitis C infection
Synthesis
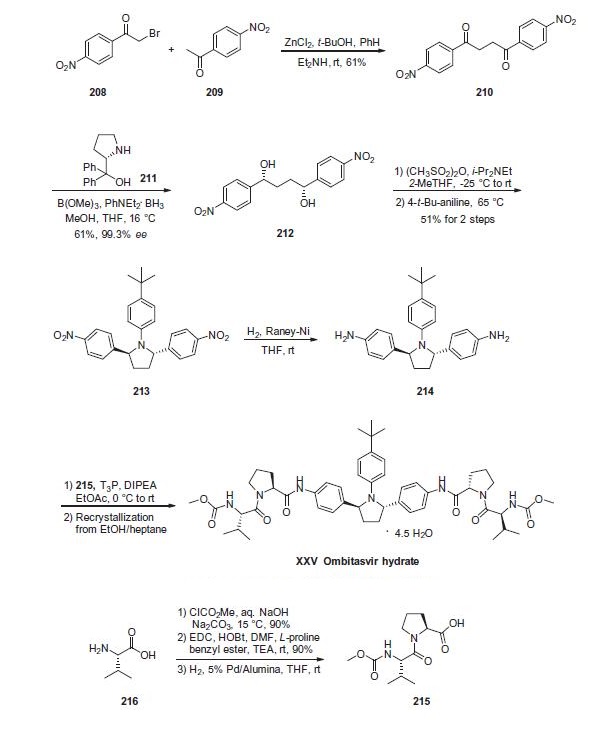
Alkylation of 1-(4-nitrophenyl)ethanone (209) with 2-bromo-1-(4-nitrophenyl)ethanone (208) in the presence of zinc chloride produced diketone 210 in 61% yield. Asymmetric reduction of the diketone using N,N-diethylaniline borane with (S)-(-)-a,a-diphenyl-2-pyrrolidinemethanol (211) and trimethoxyborate gave diol 212 in 61% yield and 99.3% ee. The diol was then treated with methanesulfonic anhydride to generate the corresponding bis-mesylate which was reacted with 4-tert-butylaniline to give pyrrolidine 213 in 51% yield over the two steps. Hydrogenolysis of the nitro groups was accomplished using Raney nickel catalyst to give bis-aniline 214. Separately, (L)-valine (216) was reacted with methyl chloroformate to give the corresponding methyl carbamate in 90% yield which was coupled to L-proline benzyl ester in the presence of EDC and HOBt to give the corresponding dipeptide in 90% yield. Hydrogenolysis of the benzyl ester group of the protected dipeptide using Pd/alumina catalyst produced dipeptide acid 215. Aniline 214 was treated with two equivalents of acid 215 in the presence of 1-propanephosphonic acid cyclic anhydride (T3P). The crude product was recrystallized from ethanol and heptane to give ombitasvir hydrate (XXV). No yields were provided to the final steps of this synthesis.
Enzyme inhibitor
This N-phenylpyrrolidine-based, pan-genotypic HCV NS5A protease inhibitor (FW = 893.14 g/mol), also named ABT-267, targets Nonstructural protein 5A (NS5A), a zinc-binding and proline-rich hydrophilic phosphoprotein that plays a key role in Hepatitis C virus RNA replication.Ombitasvir exhibits low-picomolar EC50 values against Hepatitis C virus, with superior pharmacokinetics. Although ombitasvir shows a low barrier to resistance, when given as monotherapy, co-administration with other antivirals enhances its barrier to resistance. Indeed, a 12-week, Phase- 3 study demonstrated that a multi-targeted regimen consisting of ombitasvir, dasabuvir and ribavirin is highly effective and showed a low rate of treatment discontinuation.
Drug interactions
Potentially hazardous interactions with other drugs
See also ritonavir interactions.
Ombitasvir:
Antibacterials: concentration possibly reduced by
rifampicin - avoid.
Antidepressants: concentration possibly reduced by
St John’s wort - avoid.
Antiepileptics: concentration reduced by
carbamazepine - avoid, concentration possibly
reduced by fosphenytoin, phenobarbital, phenytoin
and primidone - avoid.
Diuretics: concentration of furosemide reduced
(reduce furosemide dose).
Immunosuppressants: increases concentration of
ciclosporin (reduce ciclosporin dose by a fifth);
everolimus (avoid); sirolimus and tacrolimus (reduce
dose and use only if benefit outweighs risk - see
SPC).
Oestrogens: avoid with ethinyloestradiol.
Statins: avoid with atorvastatin and simvastatin.
Paritaprevir:
Antibacterials: avoid with clarithromycin;
concentration possibly reduced by rifampicin -
avoid.
Antidepressants: concentration possibly reduced by
St John’s wort - avoid.
Antiepileptics: concentration reduced by
carbamazepine - avoid; possibly reduced by
fosphenytoin, phenobarbital, phenytoin and
primidone - avoid.
Antifungals: concentration of both drugs increased
with ketoconazole and possibly itraconazole and
posaconazole - avoid.
Antivirals: concentration increased by atazanavir;
concentration increased by darunavir and
concentration of darunavir decreased; avoid with
efavirenz, etravirine, indinavir, nevirapine, saquinavir
and tipranavir; concentration increased by lopinavir
- avoid.
Diuretics: concentration of furosemide increased
(reduce furosemide dose).
Immunosuppressants: increases concentration of
ciclosporin (reduce ciclosporin dose by a fifth);
everolimus (avoid); sirolimus and tacrolimus (reduce
Antidepressants: concentration possibly reduced by
St John’s wort - avoid.
Antiepileptics: concentration reduced by
carbamazepine - avoid; possibly reduced by
fosphenytoin, phenobarbital, phenytoin and
primidone - avoid.
Antifungals: concentration of both drugs increased
with ketoconazole and possibly itraconazole and
posaconazole - avoid.
Antivirals: concentration increased by atazanavir;
concentration increased by darunavir and
concentration of darunavir decreased; avoid with
efavirenz, etravirine, indinavir, nevirapine, saquinavir
and tipranavir; concentration increased by lopinavir
- avoid.
Diuretics: concentration of furosemide increased
(reduce furosemide dose).
Immunosuppressants: increases concentration of
ciclosporin (reduce ciclosporin dose by a fifth);
everolimus (avoid); sirolimus and tacrolimus (reduce
Metabolism
Ombitasvir is metabolised via amide hydrolysis followed
by oxidative metabolism. A total of 13 metabolites
were identified in human plasma. These metabolites
are not expected to have antiviral activity or off-target
pharmacologic activity.
Paritaprevir is metabolised mainly by CYP3A4 and to
a lesser extent CYP3A5. Following administration of
a single 200 mg / 100 mg oral dose of 14C paritaprevir
/ ritonavir to humans, the parent drug was the major
circulating component, accounting for approximately 90%
of the plasma radioactivity.
At least 5 minor metabolites
of paritaprevir have been identified in circulation that
accounted for approximately 10% of plasma radioactivity.
These metabolites are not expected to have antiviral
activity.
Ritonavir is extensively metabolised in the liver mainly by
cytochrome P450 isoenzymes CYP3A4 and to a lesser
extent by CYP2D6. Five metabolites have been identified and the major metabolite has antiviral activity, but
concentrations in plasma are low.
About 86% of a dose is eliminated through the faeces
(both as unchanged drug and as metabolites) and about
11% is excreted in the urine.
ABT-267 Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| CONTIDE BIOTECH CO.,LTD | +85253358525 | xena@healthtide-api.com | China | 538 | 58 |
| Nanjing Finetech Chemical Co., Ltd. | 025-85710122 17714198479 | sales@fine-chemtech.com | CHINA | 885 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32686 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hangzhou Cyanochem Co., Ltd. | +86 17788583750 | sales@cyanochem.com | CHINA | 283 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6313 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
| sgtlifesciences pvt ltd | +8617013299288 | dj@sgtlifesciences.com | China | 12382 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6393 | 58 |
View Lastest Price from ABT-267 manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-05-06 | ABT-267
1258226-87-7
|
US $0.00 / G | 1G | 99% | 20 | CONTIDE BIOTECH CO.,LTD | |
 |
2021-07-02 | ombitasvir
1258226-87-7
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2019-07-06 | ABT-267
1258226-87-7
|
US $1.00 / KG | 1G | 98% | 100KG | Career Henan Chemical Co |
-

- ABT-267
1258226-87-7
- US $0.00 / G
- 99%
- CONTIDE BIOTECH CO.,LTD
-

- ombitasvir
1258226-87-7
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-

- ABT-267
1258226-87-7
- US $1.00 / KG
- 98%
- Career Henan Chemical Co