ChemicalBook >> CAS DataBase List >>(R,R)-DIPAMP
(R,R)-DIPAMP
- CAS No.
- 55739-58-7
- Chemical Name:
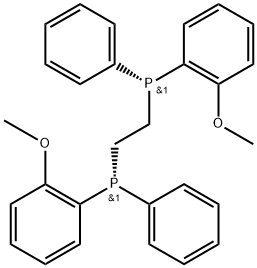
- (R,R)-DIPAMP
- Synonyms
- R)-DIPAMP;(-)-DIPAMP;(R,R)-DIPAMP;(R,R)-Dipamp ,0%;(R,R)-DIPAMP,97%;Ethylenebis(2-methoxyphenylphenylphosphine);Ethylenebis[phenyl(2-methoxyphenyl)phosphine];Ethylenebis[(2-methoxyphenyl)phenylphosphine];(R,R)-Diphenylbis-[(o-anisyl)-methylenephosphine];(1R,2R)-Bis[(2-methoxypheny)phenylphosphino]ethane
- CBNumber:
- CB0300441
- Molecular Formula:
- C28H28O2P2
- Molecular Weight:
- 458.48
- MDL Number:
- MFCD05863546
- MOL File:
- 55739-58-7.mol
Last updated:2023-05-11 17:26:00
| Melting point | 102-104 °C |
|---|---|
| Boiling point | 580.2±45.0 °C(Predicted) |
| alpha | -88o (C=1 IN CHLOROFORM) |
| refractive index | -85 ° (C=1, CHCl3) |
| form | Crystalline Powder |
| color | White |
| optical activity | [α]22/D 81°, c = 1 in chloroform |
| CAS DataBase Reference | 55739-58-7 |
| FDA UNII | BLJ831OWLW |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335-H413 | |||||||||
| Precautionary statements | P273-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 37/39-26-36/37 | |||||||||
| WGK Germany | 3 | |||||||||
| TSCA | No | |||||||||
| HS Code | 29319090 | |||||||||
| NFPA 704 |
|
(R,R)-DIPAMP Chemical Properties,Uses,Production
Uses
Rhodium DIPAMP catalysts have shown high activity and enantioselectivity in the asymmetric hydrogenation of enamides, enol acetates and olefins.
Chemical Properties
White crystalline powder
Uses
(R, R)-DIPAMP can be used:
- As a catalyst in the enantioselective [3+2] cycloaddition of γ-substituted allenoates with β-perfluoroalkyl enones and 3-butynoates with electron-deficient olefins to yield substituted cyclopentenes.
- To prepare its rhodium metal complexes, which are used as catalysts in asymmetric hydrogenation reactions.
Uses
(R,R)-DIPAMP is a reactant used in the synthesis of Rhodium diphosphine trinuclear complexes.
(R,R)-DIPAMP Preparation Products And Raw materials
Raw materials
1of5
Preparation Products
55739-58-7((R,R)-DIPAMP)Related Search:
(1R,2R)-BIS[(2-METHOXYPHENYL)PHENYLPHOSPHINO]ETHANE
[(1R,2R)-(-)-BIS[(2-METHOXYPHENYL)PHENYLPHOSPHINO]ETHANE]
(R,R)-(-)-1,2-Bis[(2-methoxyphenyl)(phenyl)phosphino]ethane,98%(-)-DIPAMP
(R,R)-(-)-1,2-BIS[2-METHOXYPHENYL)(PHENYL)PHOSPHINO]ETHANE (-)-DIPAMP
(R,R)-Diphenylbis-[(o-anisyl)-methylenephosphine]
(R,R)-Ethylenebis[(2-methoxyphenyl)phenylphosphine], [(1R,2R)-(-)-Bis[(2-methoxyphenyl)phenylphosphino]ethane], (R,R)-1,2-Ethanediylbis[(2-methoxyphenyl)phenylphosphine], (R,R)-1,2-Bis[(2-methoxyphenyl)(phenylphosphino)]ethane
Ethylenebis(2-methoxyphenylphenylphosphine)
Ethylenebis[(2-methoxyphenyl)phenylphosphine]
Ethylenebis[phenyl(2-methoxyphenyl)phosphine]
(1R,2R)-Bis[(2-methoxyphenyl)phenylphosphino]ethane,90%
(R,R)-1,2-Ethanediylbis[(2-methoxyphenyl)phenylphosphine]
(R,R)-Dipamp ,0%
(R,R)-DIPAMP,(R,R)-1,2-Bis[(2-methoxyphenyl)(phenylphosphino)]ethane, (R,R)-1,2-Ethanediylbis[(2-methoxyphenyl)phenylphosphine], (R,R)-Ethylenebis[(2-methoxyphenyl)phenylphosphine], [(1R,2R)-()-Bis[(
(R,R)-Ethylenebis[(2-methoxyphenyl)phenylphosphine]
(R,R)-DIPAMP
(-)-DIPAMP
(R,R)-(-)-1,2-Bis[(2-Methoxyphenyl)(phenyl)phosphino]ethane,(R,R)-DIPAMP
(R,R)-ETHYLENEBIS[(2-METHOXYPHENYL)PHENYLPHOSPHINE]
(R,R)-DIPAMP
(R,R)-(-)-1,2-BIS[(2-METHOXYPHENYL)(PHENYL)PHOSPHINO]ETHANE
1,2-Bis((R)-(2-Methoxyphenyl)(phenyl)phosphino)ethane
(R,R)-(-)-1,2-Bis[(2-methoxyphenyl)(phenyl)phosphino]ethane,98% (R,R)-DIPAMP
(1R,2R)-Bis[(2-methoxypheny)phenylphosphino]ethane
R)-DIPAMP
(R,R)-1,2-Bis[(2-methoxyphenyl)phenylphosphino]ethane>
Phosphine, 1,1'-(1,2-ethanediyl)bis[1-(2-methoxyphenyl)-1-phenyl-, (1R,1'R)-rel-
(R,R)-DIPAMP,97%
1,2-bis((R)-(2-methoxyphenyl)(phenyl)phosphanyl)ethane
55739-58-7
C6H4OCH3C6H5PCH2CH2PC6H4OCH3C6H5
C28H28O2P2
CH3OC6H4PC6H5CH22
Chiral Catalysts, Ligands, and Reagents
Asymmetric Synthesis
Privileged Ligands
Chiral Phosphine
Asymmetric Synthesis
Phosphine Ligands
Synthetic Organic Chemistry
organophosphine ligand