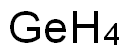GERMANE
- CAS No.
- 7782-65-2
- Chemical Name:
- GERMANE
- Synonyms
- GeH4;Un2192;GERMANE;Monogermane;GeH4 99,999%;GERMANE 99.99+%;Einecs 231-961-6;germaniumhydride;Germanium hydride;Deuterated Germane
- CBNumber:
- CB0317847
- Molecular Formula:
- GeH4
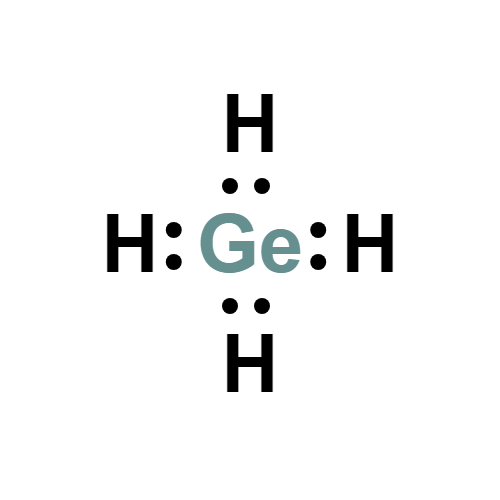
Lewis structure
- Molecular Weight:
- 76.64
- MDL Number:
- MFCD00011028
- MOL File:
- 7782-65-2.mol
- MSDS File:
- SDS
| Melting point | -165°C |
|---|---|
| Boiling point | -88°C |
| Density | 1,53 g/cm3 |
| vapor density | 1.53 (142 °C, vs air) |
| solubility | insoluble in H2O |
| form | colorless gas |
| Specific Gravity | 1.53 |
| color | colorless gas; flammable |
| Water Solubility | insoluble H2O; soluble liquid ammonia, slightly soluble hot HCl [HAW93] |
| Hydrolytic Sensitivity | 10: reacts extremely rapidly with moisture and oxygen - may be pyrophoric - sealed system required |
| Merck | 13,4418 |
| Exposure limits | TLV-TWA 0.62 mg/m3 (0.3 ppm) (ACGIH). |
| CAS DataBase Reference | 7782-65-2 |
| EWG's Food Scores | 1 |
| FDA UNII | 619P6J82AE |
| EPA Substance Registry System | Germane (7782-65-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H220-H280-H302-H330 | |||||||||
| Precautionary statements | P210-P260-P284-P310-P410+P403 | |||||||||
| Hazard Codes | F,T,T+,F+ | |||||||||
| Risk Statements | 17-21/22-23-26-22-12 | |||||||||
| Safety Statements | 16-24-26-36/37/39-45 | |||||||||
| RIDADR | 2192 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | LY4900000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 2.3 | |||||||||
| NFPA 704 |
|
GERMANE Chemical Properties,Uses,Production
Introduction
Germanium forms several tetravalent hydrides that have the general formula GenH2n+2 similar to alkanes and silicon hydrides. The formulas and CAS Registry numbers of the three common hydrides are:
Name CAS No. Formula
Monogermane (the tetrahydride) [7782-65-2] GeH4
Digermane [13818-89-8] Ge2H6
Trigermane [14691-44-2] Ge3H8
Monogermane is used to produce high purity germanium metal. It also is used as a doping substance for electronic components.
Reaction
Germanium hydrides are less stable than the corresponding hydrides of carbon and silicon. Thermal decomposition produces germanium and hydrogen. Monogermane decomposes at 350°C, while digermane and trigermane decompose to their elements at 210° and 190°C, respectively, at 200 torr. At elevated temperatures the hydrides dissociate, depositing mirror-like germanium crystals on container surfaces. Heating with oxygen yields germanium oxide. GeO2:
GeCl4 + 2O2→GeO2 + 2H2O
Preparation
Polygermanes may be prepared by the reaction of magnesium germanide, Mg2Ge, with dilute hydrochloric acid in an atmosphere of hydrogen. Monogermane, GeH4, may be prepared by various methods, such as: (1) Reduction of germanium tetrachloride, GeCl4, with lithium aluminum hydride in ether, (2) Electrolysis of a solution of germanium oxide, GeO2, in sulfuric acid using lead electrodes, and (3) Reaction of magnesium germanide and ammonium bromide, NH4Br, in liquid ammonia.
Toxicity
Monogermane is moderately toxic. Inhalation causes irritation of the respiratory tract. Chronic exposure can induce kidney and liver damage.
Chemical Properties
Colorless gas, decomposes at 350C, insoluble in water, soluble in liquid ammonia, slightly soluble in hot hydrochloric acid.
Chemical Properties
Germane is a colorless, flammable gas. Pungent odor.
Uses
Doping agent for solid-state electronic components
Uses
Germanium tetrahydride (GeH4) is used to produce crystals of germanium. It is extremely toxic.
Uses
It is used to produce high-purity germaniummetal and as a doping substance for electroniccomponents.
Definition
A germanium hydride of the general formula GenH2n+2.
General Description
GERMANE is a colorless gas with a pungent odor.The gas is heavier than air and a flame can flash back to the source of leak very easily. GERMANE is toxic by inhalation. Prolonged exposure of the containers to fire or intense heat may result in their violent rupturing and rocketing. GERMANE is used in making electronics.
Air & Water Reactions
Highly flammable. Pyrophoric, the germanium hydrides are spontaneously flammable in air [Merck 1989]. Germanium has an exothermic reaction when dropped in water accompanied by crackling [Bretherick's 5th edition].
Reactivity Profile
Hydrides, such as GERMANE, are reducing agents and react rapidly and dangerously with oxygen and with other oxidizing agents, even weak ones. Thus, they are likely to ignite on contact with alcohols. Hydrides are incompatible with acids, alcohols, amines, and aldehydes.
Health Hazard
TOXIC; may be fatal if inhaled or absorbed through skin. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Health Hazard
Germane is a moderately toxic gas. Itexhibits acute toxicity, lower than that ofstannane, but much greater than that ofsilane. By contrast, its poisoning effects aresomewhat similar to the group VB metalhydrides, arsine, and stibine, while beingmuch less toxic than the latter two compounds.Exposure to this gas can cause injuryto the kidney and liver. A 1-hour exposure toa concentration of 150–200 ppm in air wasfatal to test animals, including mice, guineapigs, and rabbits. Inhalation of the gas canalso cause irritation of the respiratory tract.
Fire Hazard
Flammable; may be ignited by heat, sparks or flames. May form explosive mixtures with air. Vapors from liquefied gas are initially heavier than air and spread along ground. Vapors may travel to source of ignition and flash back. Some of these materials may react violently with water. Cylinders exposed to fire may vent and release toxic and flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket. Runoff may create fire or explosion hazard.
Safety Profile
Poison by inhalation. Moderately toxic by ingestion. A hemolytic gas. Ignites spontaneously in air. Incompatible with Brz. See also HYDRIDES, GERMANIUM COMPOUNDS, and GERMANIUM.
Potential Exposure
This material is used as a doping agent in solid state electronic component manufacture.
Shipping
UN2192 Germane, Hazard Class: 2.3; Labels: 2.3-Poisonous gas, 2.1-Flammable gas, Inhalation Hazard Zone B. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.
Incompatibilities
Pyrophoric; may ignite spontaneously in air. Attacks hydrocarbon and fluorocarbon lubricants. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from oxidizing and nonoxidizing acids, ammonia, aqua regia, sulfuric acid, carbonates, halogens, and nitrates. Explosive reaction or ignition with potassium chlorate, potassium nitrate, chlorine, bromine, oxygen, and potas sium hydroxide in the presence of heat.
Waste Disposal
Return refillable compressed gas cylinders to supplier. Dispose of contents and container to an approved waste disposal plant. All federal, state, and local environmental regulations must be observed.
GERMANE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9409 | 58 |
| Meryer (Shanghai) Chemical Technology Co., Ltd. | 4006608290; 18621169109 | market03@meryer.com | China | 40241 | 62 |
| Energy Chemical | 021-021-58432009 400-005-6266 | sales8178@energy-chemical.com | China | 44751 | 61 |
| Zibo Zeno Pharmaceutical Technology Co., Ltd. | 0533-8800999 13515338377 | zenuoyiyao@163.com | China | 55 | 58 |
| Central China Special Gas (CCSG) Co., Ltd | 0734-8755555 15674722888 | lyq@ccsg.cn | China | 281 | 58 |
| TCI (Shanghai) Chemical Trading Co., Ltd. | 021-61109150 | sales@tcisct.com | China | 31183 | 58 |
| DWS Specialty Gas Co., Ltd | 159-0619-7626 13194677939 | yanning@abamtc.com | China | 454 | 58 |
| Fuxin Pharmaceutical | 021-50872116 13611907556 | contact@fuxinpharm.com | China | 1575 | 58 |
7782-65-2(GERMANE)Related Search:
1of4