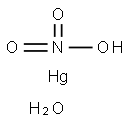Mercury nitrate monohydrate
- CAS No.
- 7783-34-8
- Chemical Name:
- Mercury nitrate monohydrate
- Synonyms
- MERCURIC NITRATE;MERCURY(II) NITRATE;MercuricNitrateExtraPure;MERCURIC NITRATE HYDRATE;Mercuric nitrate Standard;MERCURY(II) NITRATE 1 H2O;Mercuric nitrate, GR 99%+;MercuricNitrate(Hydrate)Gr;MERCURIC NITRATE N-HYDRATE;MERCURIC NITRATE 1HYD GRAN
- CBNumber:
- CB0355067
- Molecular Formula:
- H3HgNO4
- Molecular Weight:
- 281.62
- MDL Number:
- MFCD00149736
- MOL File:
- 7783-34-8.mol
- MSDS File:
- SDS
| Melting point | 79 °C(lit.) |
|---|---|
| Density | 1.025 g/mL at 25 °C |
| vapor density | 11 (vs air) |
| storage temp. | Store at RT. |
| solubility | soluble in H2O, dilute acid solutions |
| form | Liquid |
| Specific Gravity | 4.39 |
| color | White to off-white |
| Odor | Slight nitric acid odor |
| Water Solubility | Soluble in water. |
| Sensitive | Hygroscopic |
| Merck | 14,5880 |
| Exposure limits |
ACGIH: TWA 0.025 mg/m3 (Skin) NIOSH: IDLH 10 mg/m3; TWA 0.05 mg/m3; Ceiling 0.1 mg/m3 |
| CAS DataBase Reference | 7783-34-8(CAS DataBase Reference) |
| FDA UNII | YJ09EDI5UY |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300+H310+H330-H373-H410 | |||||||||
| Precautionary statements | P262-P273-P280-P301+P310+P330-P302+P352+P310-P304+P340+P310 | |||||||||
| Hazard Codes | T+,N,T,O,Xn | |||||||||
| Risk Statements | 26/27/28-33-50/53-34-23/24/25-8-20/21/22-51/53-52/53 | |||||||||
| Safety Statements | 13-28-45-60-61-36/37/39-26-17-36/37 | |||||||||
| RIDADR | UN 1625 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | OW8225000 | |||||||||
| F | 3-8 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28521000 | |||||||||
| Toxicity | LD50 orally in Rabbit: 26 mg/kg LD50 dermal Rat 75 mg/kg | |||||||||
| NFPA 704 |
|
Mercury nitrate monohydrate price More Price(30)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 1.04439 | Mercury(II) nitrate monohydrate foranalysisEMSURE?ACS,Reag.PhEur | 7783-34-8 | 50g | $187 | 2022-05-15 | Buy |
| Sigma-Aldrich | 1.04439 | Mercury(II) nitrate monohydrate foranalysisEMSURE?ACS,Reag.PhEur | 7783-34-8 | 250g | $370 | 2022-05-15 | Buy |
| Sigma-Aldrich | 230421 | Mercury(II) nitrate monohydrate ACS reagent, ≥98.0% | 7783-34-8 | 250g | $306 | 2021-12-16 | Buy |
| Sigma-Aldrich | 230421 | Mercury(II) nitrate monohydrate ACS reagent, ≥98.0% | 7783-34-8 | 50g | $97.2 | 2021-12-16 | Buy |
| Alfa Aesar | 014497 | Mercury(II) nitrate hydrate, ACS, 98.0% min | 7783-34-8 | 50g | $67 | 2023-06-20 | Buy |
Mercury nitrate monohydrate Chemical Properties,Uses,Production
Chemical Properties
The monohydrate is a white crystalline or powdery substance; density 4.3g/cm3; decomposes on heating; soluble in water and nitric acid; insoluble in alcohol.
It is stable and hygroscopic. Mercuric nitrate is incompatible with mercuric nitrate in contact with organic materials, powdered metals, petroleum hydrocarbons, hypophosphoric acid, unsaturates, and aromatics, which react violently.
Uses
Mercury(II) nitrate is used in the preparation of other mercury compounds; in organic synthesis; and as an analytical standard for mercury.
Preparation
Mercury(II) nitrate is prepared by dissolving mercury in excess hot concentrated nitric acid. Upon evaporation of the solution, large colorless deliquescent crystals of monohydrate, Hg(NO3)2•H2O, form.
It also is obtained by boiling a solution of mercury(I) nitrate or by the action of light on mercury(I) nitrate:
Hg2(NO3)2 → Hg + 2HgNO3
Reactions
Gentle heating of mercury(II) nitrate gives mercury(II) oxide evolving nitrogen and oxygen:
Hg(NO3)2 → HgO + 2NO2 + ½ O2
However, on strong heating, mercury nitrate decomposes to mercury metal:
Hg(NO3)2 → Hg + 2NO2 + O2
When excess alkali hydroxide is added to a solution of mercury(II) nitrate, a yellow precipitate of HgO is obtained.
Addition of potassium thiocyanate solution forms a white precipitate of mercury(II) thiocyanate:
Hg2+ + 2SCN¯ → Hg(SCN)2
Addition of a small amount of alkali iodide to mercury(II) nitrate solution precipitates mercury(II) iodide:
Hg2+ + 2I¯ → HgI2
Similarly, mercury(II) cyanide precipitates upon the addition of potassium cyanide to mercury(II) nitrate solution:
Hg2+ + 2CN¯ → Hg(CN)2
Toxicity
Mercury(II) nitrate is highly toxic by ingestion and possibly other routes of exposure. The LD50 oral for the dihydrate in mouse is 25 mg/kg.
Chemical Properties
Mercuric nitrate is a white to yellowish crystalline solid with an odor like nitric acid. Normally exists as the hemihydrate or the dihydrate
Uses
Mercury(II) nitrate is used as an oxidizing agent involved in organic synthesis. Its reaction with acetone yielded an organometallic compound containing mercury. In addition, it serves as a nitrification agent and analytical reagents used in the laboratory. It is involved in the preparation of mercury fulminate.
Potential Exposure
Mercuric nitrate is used in making other chemicals; in felt manufacture and in making mercury fulminate
Shipping
UN1625 Mercuric nitrate, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Its solubility in H2O containing 1% HNO3 is 7.7%. Recrystallise it from a warm saturated solution of dilute HNO3 and cool to room temperature slowly to give elongated prisms. Rapid cooling gives plates. The colourless crystals should be stored in the dark. POISONOUS. [Grdenic J Chem Soc 1312 1956.]
Incompatibilities
A strong oxidizer. Reacts violently with combustibles, petroleum hydrocarbons; reducing agents; aldehydes, ammonia, ketones, phosphorus. Reacts with acetylene, alcohol, phosphine, and sulfur to form shocksensitive compounds. Aqueous solution attacks most metals. Vigorous and dangerous reaction with petroleum hydrocarbons. Incompatible with organic materials; acetylene, ethanol, phosphine, sulfur, hypophosphoric acid. Inorganic mercury compounds are incompatible with acetylene, ammonia, chlorine dioxide; azides, calcium (amalgam formation), sodium carbide; lithium, rubidium, copper. Decomposes in heat or on exposure to light, producing toxic fumes (mercury, nitrogen oxides)
Mercury nitrate monohydrate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Qiuxian Baitai New Material Co., LTD | +8618330912755 | sale2@hbyalin.com | China | 1677 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9358 | 58 |
| GLR Innovations | +91 9891111994 | info@glrgroup.in | India | 4542 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24738 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33349 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
View Lastest Price from Mercury nitrate monohydrate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-08-12 | MERCURIC NITRATE, MONOHYDRATE
7783-34-8
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- MERCURIC NITRATE, MONOHYDRATE
7783-34-8
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
7783-34-8(Mercury nitrate monohydrate)Related Search:
1of4