Cyclopropane
- CAS No.
- 75-19-4
- Chemical Name:
- Cyclopropane
- Synonyms
- TRIMETHYLENE;Cyclopropnane;CYCLOPROPANE;CYCLOPROPANE, 99+%;trimethylene(cyclic);Trimethylene (cyclic);cyclopropane,liquefied;Cyclopropane ISO 9001:2015 REACH
- CBNumber:
- CB0368506
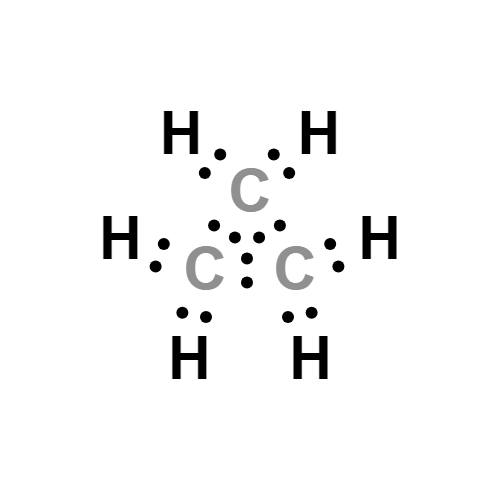
- Molecular Formula:
- C3H6
Lewis structure
- Molecular Weight:
- 42.08
- MDL Number:
- MFCD00001268
- MOL File:
- 75-19-4.mol
- MSDS File:
- SDS
| Melting point | −128 °C(lit.) |
|---|---|
| Boiling point | −33 °C(lit.) |
| Density | 0.6190 |
| vapor density | 1.45 (vs air) |
| refractive index | 1.3799 |
| pka | 46(at 25℃) |
| explosive limit | 10.4% |
| Water Solubility | 464mg/L(25 ºC) |
| Merck | 13,2777 |
| InChIKey | LVZWSLJZHVFIQJ-UHFFFAOYSA-N |
| CAS DataBase Reference | 75-19-4 |
| EWG's Food Scores | 1 |
| FDA UNII | 99TB643425 |
| EPA Substance Registry System | Cyclopropane (75-19-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS04 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H220-H280 | |||||||||
| Precautionary statements | P210-P377-P381-P410+P403 | |||||||||
| Hazard Codes | F+ | |||||||||
| Risk Statements | 12 | |||||||||
| Safety Statements | 9-16-33 | |||||||||
| RIDADR | UN 1027 2.1 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | GZ0690000 | |||||||||
| Autoignition Temperature | 928 °F | |||||||||
| HazardClass | 2.1 | |||||||||
| HS Code | 2903898090 | |||||||||
| Toxicity | LCLo inhalation in mouse: 282gm/m3/2H | |||||||||
| NFPA 704 |
|
Cyclopropane price More Price(7)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 295183 | Cyclopropane ≥99% | 75-19-4 | 25g | $1100 | 2024-03-01 | Buy |
| American Custom Chemicals Corporation | CHM0156916 | CYCLOPROPANE 95.00% | 75-19-4 | 25G | $1286.73 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | CHM0156916 | CYCLOPROPANE 95.00% | 75-19-4 | 100G | $4095 | 2021-12-16 | Buy |
| SynQuest Laboratories | 1200-1-03 | Cyclopropane 99% | 75-19-4 | 5G | $75 | 2021-12-16 | Buy |
| Apolloscientific | OR55510 | Cyclopropane 99% | 75-19-4 | 5g | $174 | 2021-12-16 | Buy |
Cyclopropane Chemical Properties,Uses,Production
Chemical Properties
Cyclopropane is the simplest possible cyclical hydrocarbon. Though saturated, its cyclical structure endows it with some of the properties associated with unsaturated compounds. It is relatively stable chemically, does not isomerize or polymerize during prolonged storage under normal conditions, and does not react with soda lime in the presence of heat. The gas is colourless, sweet smelling and, in concentrations in excess of 50 percent, is slightly irritant to respiratory mucosa. The molecular weight of the gas is 42.08 and the vapour density (air=1.0) is 1-75 at 20°C (Macintosh, Mushin and Epstein, 1963). The boiling point of liquid cyclopropane is -33°C at a pressure of 760 Torr, and the critical temperature is 125°C. The critical pressure is 54 atmospheres, but at room temperature the gas liquefies when exposed to pressures of 5 lb./sq.in.
As supplied, the gas is 99.5 percent or more pure, but possible impurities include propylene, allene, cyclohexane, nitrogen, carbon dioxide, and bromor chloropropane. Propylene is the most important contaminant, up to 1 per cent being acceptable, but concentrations over 3 per cent are toxic.
History
Cyclopropane was discovered by Freund (1882), but the anaesthetic properties of hydrocarbons with the general formula CnH2n were appreciated earlier. John Snow (1858), for example, administered amylene (C5H10) to 238 patients. In 1923, ethylene (C2H4) was introduced into clinical anaesthesia, but its low potency and inflammability restricted its use. At the University of Toronto, research by G. H. W. Lucas and V. E. Henderson on propylene (CH3.CH:CH2) revealed certain samples to show marked cardiac toxicity. Lucas suggested that the toxicity might arise from one of the impurities present in the samples, the cyclic isomer of propylene, cyclopropane. Pure samples of cyclopropane were prepared and this, when tested by Lucas and Henderson, proved to be a more potent and suitable anaesthetic agent than propylene.
Solubility constants
Cyclopropane is sparingly soluble in water, but strongly lipophilic. This is shown by a water/gas partition coefficient of 0-204 (Orcutt and Seevers, 1937) and an oil/gas coefficient of 11-2 (Blumberg et al., 1952). The strong lipoid and protein afiBnities of the gas lead not only to the carriage of 2-5 times more cyclopropane by red cells than plasma, but also to a blood/gas partition coefficient which varies with both the haemoglobin concentration and the plasma fat content of blood (Robbins, 1958). The Ostwald solubility coefficient for cyclopropane and whole blood at 37℃, and with 15g Hb per 100 ml, is 0-415 (Posati and Faulconer, 1958).
Uses
Cyclopropane is a powerful, nonirritating agent which can produce anaesthesia in a few breaths. It has been used used as an anesthetic, but this use has been discontinued because the gas is dangerous to manufacture and handle.
Cyclopropane has been used as the pseudo-π unsaturated hydrocarbon prototype to study the rotational spectrum of its complex formed with chlorine monofluoride and the similarity of this complex with isotopomers.
Reactions
Cyclopropane and cyclobutane are gases at ordinary temperatures; the remaining cycloalkanes are liquids. Their melting points and boiling points show a gradual rise with the increase in molecular weight.
Addition of Cl2 and Br2: Cydopropane reacts with Cl, and Br, at room temperature and in the absence of diffused sunlight to produce 1,3- dichlorocyclopropane and 1,3-dibromocyclopropane respective.
Cyclopropane reacts with HBr and HI to give 1-bromopropane and 1-iodopropane.
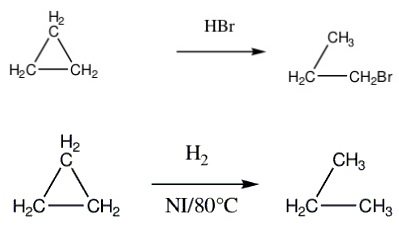
Cyclopropane and cyclobutane react with hydrogen in the presence of a nickel catalyst to produce propane and n-butane respectively.
Preparation
Stroll quantities of cyclopropane have been prepared from natural deposits of the gas in the United States, but current practice involves the reaction of trimethylene glycol with hydrobromic acid to form1,3-Dibromopropane. The latter compound, in the presence of zinc dust, converts to the cyclic compound, cyclopropane.
Description
Cyclopropane (molecular formula: C3H6) is a kind of cycloalkane molecule. Cyclopropane and propene are isomers of each other. It can be used as a kind of anaesthetic. However, due to its side effect of causing a sudden decrease in blood pressure and potentially causing cardiac dysrhythmia, it has not been available for clinical use since mid-1980 as more. Its mechanism of action is through acting as the NMDA receptor antagonist as well as inhibiting the AMPA receptor, inhibiting the nicotine acetylcholine receptor, and activates certain K2P channels.
Chemical Properties
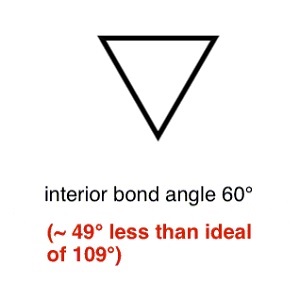
Cyclopropane is a colourless gas, C3H6, b.p. –34.5°C, whose moleculescontain a triangular ring of carbonatoms. It is made by treating 1,3-dibromopropanewith zinc metal, andis used as a general anaesthetic.
Uses
Cyclopropane was initially investigated because it was thought to be the toxic element in ethylene. Instead, it turned out to be an excellent anesthetic with very rapid onset and recovery while maintaining stable hemodynamics. Its use was ultimately limited because it was highly explosive. Cyclopropane is a sweet-smelling, flammable gas that has little or no laboratory, commercial, or industrial use.
Definition
ChEBI: Cyclopropane is a cycloalkane composed of three carbon atoms to form a ring. It has a role as an inhalation anaesthetic. It is a cycloalkane and a member of cyclopropanes.
Production Methods
Cyclopropane is prepared in reagent grade by the reduction of 1,2-dibromocyclopropane with zinc and alcohol.
General Description
Cyclopropane is a colorless gas with a petroleum-like odor. It is shipped as a liquid at 4-6 atms. It is easily ignited. The vapors are heavier than air. Contact with the liquid may cause frostbite. It can asphyxiate by the displacement of air and has a narcotic effect in high concentration (formerly used as an anesthetic gas). Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
CYCLOPROPANE is incompatible with strong oxidizing agents such as nitric acid. Boiling of the liquid and charring may occur followed by ignition of remaining material and other nearby combustibles. Adsorbed readily by concentrated sulfuric acid [Merck]. In other settings, mostly unreactive. Not affected by aqueous solutions of acids, alkalis, most oxidizing agents, and most reducing agents. When heated sufficiently or when ignited in the presence of air, oxygen or strong oxidizing agents, burns exothermically to produce carbon dioxide and water. Mixtures with oxygen or air may explode [Merck]. Contact of the cold liquefied gas with water may result in vigorous or violent boiling and extremely rapid vaporization due to the large temperature differences involved. If the water is hot, a liquid "superheat" explosion may occur. Pressures may build to dangerous levels if the liquid contacts water in a closed container, [Handling Chemicals Safely, 1980. p. 250].
Health Hazard
Inhalation causes some analgesia, anesthesia, pupil dilation, shallow depth of respirations, decreasing muscle tone. Contact with liquid may cause frostbite.
Fire Hazard
EXTREMELY FLAMMABLE. Will be easily ignited by heat, sparks or flames. Will form explosive mixtures with air. Vapors from liquefied gas are initially heavier than air and spread along ground. CAUTION: Hydrogen (UN1049), Deuterium (UN1957), Hydrogen, refrigerated liquid (UN1966) and Methane (UN1971) are lighter than air and will rise. Hydrogen and Deuterium fires are difficult to detect since they burn with an invisible flame. Use an alternate method of detection (thermal camera, broom handle, etc.) Vapors may travel to source of ignition and flash back. Cylinders exposed to fire may vent and release flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.
Safety Profile
Mutation data reported. Questionable carcinogen. High concentrations are narcotic. Human reproductive effects. Very dangerous fire hazard when exposed to heat or flame; can react with oxidizing materials. Explosion Hazard: Moderate in the form of vapor when exposed to heat or flame. To fight fire, stop flow of gas, then use CO2, dry chemical, or water spray. When heated to decomposition it emits acrid smoke and fumes.
Synthesis
Cyclopropane is manufactured commercially from 1,3-dichloropropane with zinc in the presence of sodium iodide.
Potential Exposure
Cyclopropane is used as an anesthetic and used to make other chemicals.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. If frostbite has occurred, seek medical attentionimmediately; do NOT rub the affected areas or flush themwith water. In order to prevent further tissue damage, doNOT attempt to remove frozen clothing from frostbittenareas. If frostbite has NOT occurred, immediately and thoroughly wash contaminated skin with soap and water
storage
Color Code—Red Stripe: Flammability Hazard:Store separately from all other flammable materials. ColorCode—Red: Flammability Hazard: Store in a flammableliquid storage area or approved cabinet away from ignitionsources and corrosive and reactive materials. Prior to working with cyclopropane you should be trained on its properhandling and storage. Before entering confined space wherethis gas may be present, check to make sure that an explosive concentration does not exist. Cyclopropane must bestored to avoid contact with oxidizers (such as perchlorates,peroxides, permanganates, chlorates, and nitrates) and oxygen, since violent reactions occur. Store in tightly closedcontainers in a cool, well-ventilated area away from heat orflame. Outside or detached storage is recommended. Useonly nonsparking tools and equipment, especially whenopening and closing containers of this chemical. Sources ofignition, such as smoking and open flames, are prohibitedwhere this chemical is used, handled, or stored in a mannerthat could create a potential fire or explosion hazard.Wherever this chemical is used, handled, manufactured, orstored, use explosion-proof electrical equipment and fittings. Procedures for the handling, use, and storage of cylinders should be in compliance with OSHA 1910.101 and1910.169, as with the recommendations of the CompressedGas Association
Shipping
UN1027 Cyclopropane, Hazard Class: 2.1; Labels: 2.1-Flammable gas. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written per- mission of the owner.
Purification Methods
Wash cyclopropane with a solution of HgSO4 and dry it with CaCl2, then Mg(ClO4)2. It is an anaesthetic FLAMMABLE gas and is packaged in steel cylinders. [Rifi Org Synth Coll 52 22 1972, Simmons & Smith J Am Chem Soc 80 5323 1958, Beilstein 5 H 15.]
Incompatibilities
May form explosive mixture with air. Heat, flame, or contact with oxidizers can cause fire and explosion hazard. May accumulate static electrical charges, and may cause ignition of its vapors. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materi- als, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
Return refillable compressed gas cylinders to supplier.
References
https://en.wikipedia.org/wiki/Cyclopropane
Cyclopropane Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81138252 +86-18789408387 | 1057@dideu.com | China | 3537 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9116 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| Shanghai wechem chemical co., ltd | 18824865657 | joey.lin@wechem.cn | China | 506 | 58 |
| Central China Special Gas (CCSG) Co., Ltd | 0734-8755555 15674722888 | lyq@ccsg.cn | China | 281 | 58 |
| Syntechem Co.,Ltd | info@syntechem.com | China | 12990 | 57 | |
| Shanghai Meishui Chemical Technology Co., Ltd | 021-60549325 18616193163 | China | 4528 | 56 | |
| Shanghai Yanze Chemical Co., Ltd. | 021-13773155751 19975051755 | 260924549@qq.com | China | 8554 | 58 |
View Lastest Price from Cyclopropane manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-08-12 | CYCLOPROPANE
75-19-4
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2020-01-08 | Cyclopropane
75-19-4
|
US $1.00 / KG | 1KG | 99% | 20T | Shaanxi Dideu Medichem Co. Ltd |
-

- CYCLOPROPANE
75-19-4
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-

- Cyclopropane
75-19-4
- US $1.00 / KG
- 99%
- Shaanxi Dideu Medichem Co. Ltd
75-19-4(Cyclopropane)Related Search:
1of4