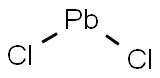Lead(II) chloride
- CAS No.
- 7758-95-4
- Chemical Name:
- Lead(II) chloride
- Synonyms
- PbCl2;LEAD CHLORIDE;Leclo;PbCI2;NA 2291;Cotunnite;ead(II) chL;dichlorolead;Bleidichlorid;leaddichloride
- CBNumber:
- CB0385798
- Molecular Formula:
- Cl2Pb
Lewis structure

- Molecular Weight:
- 278.11
- MDL Number:
- MFCD00011157
- MOL File:
- 7758-95-4.mol
- MSDS File:
- SDS
| Melting point | 501 °C(lit.) |
|---|---|
| Boiling point | 950 °C(lit.) |
| Density | 5.85 g/mL at 25 °C(lit.) |
| vapor pressure | 1 mm Hg ( 547 °C) |
| Flash point | 951°C |
| storage temp. | Store below +30°C. |
| solubility | aliphatic hydrocarbons: slightly soluble(lit.) |
| form | powder |
| color | White to off-white |
| Specific Gravity | 5.85 |
| Water Solubility | Soluble in hot water, alkali hydroxides and NH<sub>4</sub>Cl solution. Insoluble in cold water and alcohol. |
| Hydrolytic Sensitivity | 0: forms stable aqueous solutions |
| Merck | 14,5404 |
| Solubility Product Constant (Ksp) | pKsp: 4.77 |
| Exposure limits |
ACGIH: TWA 0.05 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 0.050 mg/m3 |
| Dielectric constant | 4.2(0.0℃) |
| Stability | Stable. Incompatible with strong oxidizing agents, strong acids. |
| CAS DataBase Reference | 7758-95-4(CAS DataBase Reference) |
| EWG's Food Scores | 5 |
| FDA UNII | 4IL61GN3YI |
| NIST Chemistry Reference | Lead dichloride(7758-95-4) |
| EPA Substance Registry System | Lead(II) chloride (7758-95-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302+H332-H351-H360Df-H372-H410 | |||||||||
| Precautionary statements | P201-P273-P301+P312+P330-P304+P340+P312-P308+P313 | |||||||||
| Hazard Codes | T,N | |||||||||
| Risk Statements | 61-20/22-33-50/53-62 | |||||||||
| Safety Statements | 53-45-60-61 | |||||||||
| RIDADR | UN 2291 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | OF9450000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28273990 | |||||||||
| Toxicity | MLD in guinea pigs (mg/kg): 1500-2000 orally (Tartler) | |||||||||
| NFPA 704 |
|
Lead(II) chloride price More Price(53)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.07383 | Lead(II) chloride anhydrous for synthesis | 7758-95-4 | 100g | $40.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.07383 | Lead(II) chloride anhydrous for synthesis | 7758-95-4 | 500g | $105 | 2024-03-01 | Buy |
| Sigma-Aldrich | 203572 | Lead(II) chloride 99.999% trace metals basis | 7758-95-4 | 10g | $96.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 203572 | Lead(II) chloride 99.999% trace metals basis | 7758-95-4 | 50g | $321 | 2024-03-01 | Buy |
| TCI Chemical | L0291 | Lead(II) Chloride (purified by sublimation)[for Perovskite precursor] | 7758-95-4 | 1g | $69 | 2024-03-01 | Buy |
Lead(II) chloride Chemical Properties,Uses,Production
Chemical Properties
Lead(II) chloride is a white crystals or powder. Insoluble in cold water; soluble in hot water. It is an inorganic compound with the chemical formula PbS. It is also known as galena, which is the principal ore and important compound of lead. It is one of the earliest materials to be used as a semiconductor as it tends to crystallize in sodium chloride. Lead sulfide is toxic if it is heated to decomposition, which forms lead and sulfur oxides.

Lead(II) chloride has been used in the synthesis of methyl ammonium lead iodide perovskite nanocrystals with potential application in optoelectronics due to its tuneable electronic bandgap and superior photovoltaic performance. It may be used in the preparation of mixed halide perovskite (PRV) with potential application in absorber layer of PRV solar cells. Lead chloride is used as a precursor material in the fabrication of methyl ammonium lead iodide-chloride (MAPbI3-xClx) perovskites.
Uses
Lead (II) chloride is also known as lead chloride, lead dichloride, and plumbous chloride. Lead chloride is one of the most important lead-based reagents. It occurs naturally in the form of the mineral cotunnite. The solubility of lead chloride in water is low. Lead (II) chloride is the main precursor for organometallic derivatives of lead. Lead chloride has extensive applications in industries. Lead chloride is an intermediate in refining bismuth (Bi) ore. The ore containing Bi, Pb, and Zn is first treated with molten caustic soda to remove traces of acidic elements such as arsenic and tellurium. The molten lead chloride is used in the synthesis of lead titanate (PbTiO3) and barium PbTiO3. It is used in organometallic synthesis to make metallocenes, known as plumbocenes. Lead chloride is used in production of infrared transmitting glass and in production of ornamental glass called aurene glass. This stained glass has an iridescent surface formed by spraying with lead chloride and reheating under controlled conditions. Stannous chloride (SnCl2) is used for the same purpose.
Reactions
Lead(II) chloride reacts with chlorine to produce Lead(IV) chloride: PbCl2+ Cl2→PbCl4.
Description
Lead chloride is a white crystalline powder.Molecular weight = 278.00;Boilingpoint = 950℃;Freezing/Melting point= 501C; Vapor pressure= 1 mmHgat 547℃. Hazard Identification (based on NFPA-704 MRating System): Health 2, Flammability 0, Reactivity 0.Slightly soluble in cold water; more soluble in hot water.
Chemical Properties
Lead chloride is a white crystalline powder
Physical properties
White orthorhombic crystals; refractive index 2.199; density 5.85 g/cm3; melts at 501°C; vaporizes at 950°C; partially soluble in cold water (6.73 g/L at 0°C and 9.9 g/L at 20°C); KSP 1.17x10-5 at 25°C; moderately soluble in boiling water (33.4 g/L at 100°C); slightly soluble in dilute HCl and ammonia; insoluble in alcohol.
Occurrence
Lead dichloride occurs in nature as the mineral cotunnite. The compound is used in making many basic chlorides, such as Pattison’s lead white, Turner’s Patent Yellow, and Verona Yellow, used as pigments. Also, it is used as a flux for galvanizing steel; as a flame retardant in nylon wire coatings; as a cathode for seawater batteries; to remove H2S and ozone from effluent gases; as a sterilization indicator; as a polymerization catalyst for alphaolefins; and as a co-catalyst in manufacturing acrylonitrile.
Uses
Lead (II) chloride (PbCl2) is commonly known as the mineral cotunnite.
Uses
Lead(II) chloride is used in the synthesis of lead titanate and barium lead titanate ceramics; employed in the production of infrared transmitting glass and ornamental glass (aurene glass); useful as an electrode in geophysical applications and in solar cells. Analytical reagent, preparation of lead salts, as solder and flux. Pattinson's white lead, pigment in white paint, is the basic lead chloride.
Uses
Lead dichloride occurs in nature as the mineral cotunnite. The compound is used in making many basic chlorides, such as Pattison’s lead white, Turner’s Patent Yellow, and Verona Yellow, used as pigments. Also, it is used as a flux for galvanizing steel; as a flame retardant in nylon wire coatings; as a cathode for seawater batteries; to remove H2S and ozone from effluent gases; as a sterilization indicator; as a polymerization catalyst for alphaolefins; and as a co-catalyst in manufacturing acrylonitrile.
Uses
Definition
ChEBI: An inorganic chloride consisting of two chlorine atoms covalently bound to a central lead atom.
Preparation
Lead dichloride is precipitated by adding hydrochloric acid or any chloride salt solution to a cold solution of lead nitrate or other lead(II) salt:
Pb2+ + 2Clˉ → PbCl2
Alternatively, it is prepared by treating lead monoxide or basic lead carbonate with hydrochloric acid and allowing the precipitate to settle.
.
General Description
Prepared by reacting lead(II) oxide /acetate or carbonate with HCl. In crystalline PbCl2, each atom is coordinated by nine Cl atoms, six of which lie at the apices of a trigonal prism and the remaining three beyond the centers of the three prism faces. Each Cl is coordinated by four or five Pb atoms. Upon exposure to air it form basic chlorides such as PbCl2.Pb(OH)2.
Reactivity Profile
Lead dichloride is a weak reducing agent. Interaction of Lead dichloride and calcium is explosive on warming, [Mellor, 1941, Vol. 3, 369].
Hazard
Toxic effects from ingestion may vary from low to moderate. The oral lethal dose in guinea pigs is documented as 1,500 mg/kg. (Lewis (Sr.), R. J. 1996. Sax’s Dangerous Properties of Industrial Materials, 9th ed. New York: Van Nostrand Reinhold).
Health Hazard
DUST AND FUMES. POISONOUS IF INHALED. SOLID: If swallowed, may cause metallic taste, abdominal pain, vomiting, and diarrhea.
Fire Hazard
Not flammable. POISONOUS METAL FUMES MAY BE PRODUCED IN FIRE. Toxic metal fumes. Can emit toxic metal fumes.
Flammability and Explosibility
Not classified
Potential Exposure
Used to make lead salts; lead chromate pigments; as an analytical reagent for making other chemicals; making printed circuit boards; as a solder and flux.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least 15 min, occasionally lifting upper and lower lids. Seek med-ical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, includ-ing resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medi-cal attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Note to physician: Administer saline cathartic and alenema. For relief of colic, administer antispasmodic (cal-cium gluconate, atropine, papaverine). Consider morphinesulfate for severe pain. Whole blood lead levels, circulatingplasma/erythrocyte lead concentration ratio, urine ALA,and erythrocyte protoporphyrin fluorescent microscopy mayall be useful in monitoring or assessing lead exposure.Chelating agents, such as edetate disodium calcium (CaEDTA) and penicillamine (not penicillin), are generallyuseful in the therapy of acute lead intoxication.Antidotes cand special procedures for lead: Persons with sig-nificant lead poisoning are sometimes treated with CaEDTA while hospitalized. This “chelating" drug causes arush of lead from the body organs into the blood and kid-neys, and thus has its own hazards, and must be adminis-tered only by highly experienced medical personnel undercontrolled conditions and careful observation. Ca EDTA orsimilar drugs should never be used to prevent poisoningwhile exposure continues or without strict exposure control,as severe kidney damage can result.Note to physician: For severe poisoning BAL [British Anti-Lewisite, dimercaprol, dithiopropanol (C;HgOS2)] has beenused to treat toxic symptoms of certain heavy metals poi-soning. In the case of lead poisoning it may have SOMEvalue. Although BAL is reported to have a large margin ofsafety, caution must be exercised, because toxic effects maybe caused by excessive dosage. Most can be prevented bypremedication with 1-ephedrine sulfate (CAS: 134-72-5).
storage
Personal Protective Methods: W ear protective gloves andclothing to prevent any reasonable probability of skin con-tact. Use any barrier that will prevent contam ination fromthe dust. Safety equipment suppliers/manufacturers can pro-vide recommendations on the most protective g love/cloth-ing material for your operation. All protective clothing(suits, gloves, footwear, headgear) should be clean, avail-able each day, and put on before work. Work clothingshould be HEPA vacuumed before removal. Contact lensesshould not be wor when working with this chemical. Weardust-proof chemical goggles and face shield unless fullface-piece respiratory protection is worn. Employees shouldwash immediately with soap when skin is wet or contami-nated. Provide emergency showers and eyewash.
Shipping
UN2291 Lead compounds, soluble n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required
Purification Methods
Crystallise it from distilled water at 100o (33mL/g) after filtering through sintered-glass and adding a few drops of HCl, by cooling. After three crystallisations the solid is dried under vacuum or under anhydrous HCl vapour by heating slowly to 400o. The solubility in H2O is 0.07% at ~10o, and 0.43% at ~ 100o.
Incompatibilities
A reducing agent. Violent reaction with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, and chemically active metals. Explosive with calcium 1 warming
Lead(II) chloride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| Richest Group Ltd | 18017061086 | oled@richest-group.com | CHINA | 5601 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | eric@witopchemical.com | China | 23556 | 58 |
View Lastest Price from Lead(II) chloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-08-18 | Lead(II) chloride
7758-95-4
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2021-11-09 | Lead(II) chloride
7758-95-4
|
US $9.90 / KG | 1KG | 99% | 5tons | Hebei Guanlang Biotechnology Co., Ltd. | |
 |
2021-08-12 | Lead dichloride
7758-95-4
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- Lead(II) chloride
7758-95-4
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Lead(II) chloride
7758-95-4
- US $9.90 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
-

- Lead dichloride
7758-95-4
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd