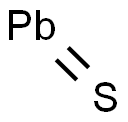Lead(II) sulfide
- CAS No.
- 1314-87-0
- Chemical Name:
- Lead(II) sulfide
- Synonyms
- p34;p128;CCNH;P-37;GALENA;ci77640;galenite;c.i.77640;C.I. 77640;p37(filter)
- CBNumber:
- CB0670186
- Molecular Formula:
- PbS
- Molecular Weight:
- 239.26
- MDL Number:
- MFCD00016280
- MOL File:
- 1314-87-0.mol
- MSDS File:
- SDS
| Melting point | 1114°C |
|---|---|
| Boiling point | 1281°C (estimate) |
| Density | 7.5 g/mL at 25 °C(lit.) |
| refractive index | 3.921 |
| storage temp. | 2-8°C |
| solubility | Soluble in strong HNO<sub>3</sub>, in excess of hot HCl |
| form | Metallic Crystals, Powder Lump |
| color | White |
| Specific Gravity | 7.5 |
| Water Solubility | Soluble in water (0.00086g/L) and acid. Insoluble in alcohol, and potassium hydroxide. |
| Crystal Structure | Cubic, Halite Structure - Space Group Fm3m |
| Merck | 14,5421 |
| Solubility Product Constant (Ksp) | pKsp: 27.1 |
| Exposure limits |
ACGIH: TWA 0.05 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 0.050 mg/m3 |
| Stability | Stable. Incompatible with oxidizing agents, acids, water. |
| InChIKey | XCAUINMIESBTBL-UHFFFAOYSA-N |
| CAS DataBase Reference | 1314-87-0(CAS DataBase Reference) |
| EWG's Food Scores | 5 |
| FDA UNII | 2425D15SYM |
| EPA Substance Registry System | Lead(II) sulfide (1314-87-0) |
| Hardness, Mohs | 2.5 |
|---|
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS02,GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H304-H315-H336-H360Df-H373-H411 | |||||||||
| Precautionary statements | P201-P210-P273-P301+P310+P331-P302+P352-P308+P313 | |||||||||
| Hazard Codes | T,N | |||||||||
| Risk Statements | 61-20/22-33-50/53-62 | |||||||||
| Safety Statements | 53-45-60-61 | |||||||||
| RIDADR | UN 3077 9/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | OG4550000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 2830908500 | |||||||||
| Toxicity | LD50 i.p. in rats: 160 mg Pb/100 g (Bradley, Fredrick) | |||||||||
| NFPA 704 |
|
Lead(II) sulfide price More Price(43)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 372595 | Lead(II) sulfide 99.9% trace metals basis | 1314-87-0 | 25g | $108 | 2024-03-01 | Buy |
| Sigma-Aldrich | 372595 | Lead(II) sulfide 99.9% trace metals basis | 1314-87-0 | 100g | $338 | 2024-03-01 | Buy |
| Sigma-Aldrich | 900738 | PbS core-type quantum dots fluorescence λem?1400?nm, 10?mg/mL in toluene | 1314-87-0 | 5ML | $362 | 2023-06-20 | Buy |
| Sigma-Aldrich | 900737 | PbS core-type quantum dots oleic acid coated, fluorescence λem?1300?nm, 10?mg/mL in toluene | 1314-87-0 | 5ML | $362 | 2023-06-20 | Buy |
| Sigma-Aldrich | 900736 | PbS core-type quantum dots fluorescence λem?1200?nm, 10?mg/mL in toluene | 1314-87-0 | 5ML | $362 | 2023-06-20 | Buy |
Lead(II) sulfide Chemical Properties,Uses,Production
Occurrence and Uses
Lead sulfide occurs in nature as the mineral galena. Most lead comes from this ore. Additionally, lead sulfide has several industrial applications. It is used in infrared detectors; transistors; photoconductive cells; high temperature lubricants; and for glazing earthenware. It also is used as a catalyst in petroleum refining for removal of mercaptans from petroleum distillates.
Physical Properties
Black powder or cubic crystal; refractive index 3.91; Moh’s hardness 2.5; melts at 1,118°C; vapor pressure 1 torr at 852°C and 5 torr at 928°C; very slightly soluble in water (124 mg/L at 20°C); KSP 9.04x10–29 at 25°C; soluble in acids.
Preparation
Lead sulfide occurs naturally as the mineral galena. It can be prepared in the laboratory as a black precipitate by passing hydrogen sulfide through a dilute acid solution of inorganic lead salt, such as lead nitrate or lead acetate:
Pb2+ + H2S → PbS + 2H+
It also is obtained by direct combination of elements by heating metallic lead with sulfur vapors.
Reactions
Lead sulfide decomposes in excess concentrated hydrochloric acid liberating hydrogen sulfide and probably forming chloroplumbus acid in solution:
PbS + 4HCl → H2PbCl4 + H2S
Two types of reactions occur with nitric acid depending on the concentration of the acid. Lead sulfide dissolves in dilute nitric acid, oxidizing to elemental sulfur:
PbS + 2HNO3 → Pb(NO3)2 + S + H2
However, treatment with concentrated nitric acid yields lead(II) sulfate:
PbS + 4HNO3 → PbSO4 + 4HNO2
Lead sulfide also undergoes various oxidation reactions at elevated temperatures that occur in a reverberatory furnace, during the production of lead from galena. Sulfur dioxide and lead sulfate are formed as intermediate products. Some typical reactions are as follows:
PbS + 2O2e→PbSO4
2PbS + 3O2→2PbO + 2SO2
PbS + 2PbO→3Pb + SO2
PbS + PbSO4→2Pb + 2SO2
When roasted in an air blast furnace, basic lead sulfate, PbO•PbSO4 (also known as sublimed white lead), is formed.
Description
Lead sulfide is a silvery to black crystallinepowder. Molecular weight = 239.25; Boilingpoint =1281℃ (sublimes); Freezing/Meltingpoint= 1114℃. Hazard Identification (based on NFPA-704M Rating System): Health 1, Flammability 0, Reactivity 0.Practically insoluble in water (0.000086 g/100-cc water at13℃).
Chemical Properties
Lead gray in color, lead-gray streak, metallic luster, good cubic cleavage. Mohs hardness 2.5. Soluble in strong nitric acid, in excess of hot hydrochloric acid.
Chemical Properties
Lead(II) sulfide is a silvery to black crystalline powder.

Lead(II) sulfide can be precipitated from a solution of lead (II) salt and hydrogen sulfide.
Lead (II) sulfide has been used during many years as source of lead (Pb). The main method to obtain the lead is the smelting of PbS and then, the lead (II) oxide obtained is reduced to Pb and carbon monoxide:
2 PbS + 3 O2 → 2 PbO + 2 SO2
PbO + C → Pb + CO
Moreover, lead (II) sulfide is used as semiconductor and photoconductor due its chemical proprieties. It is also used as black pigment. In recent years, it has been used in to obtain nanoparticles to use in electronic or electric devices.
Uses
Lead(II) sulfide is used as a semiconductor. Used in electronic devices and Infrared sensor.
Uses
The size for our lead sulfide (PbS) quantum dots (QDs) varies between 2.5 to 8 nm and depending upon this, these QDs emit between 900-1600 nm. Our PbS QDs possess high quantum yield, sharp emission and exhibit narrow fluorescence band (full width at half maximum <100 nm), which make them suitable as light absorber or IR emitter in applications in solar cells, photodetectors and infrared light emitting diodes (LEDs).
Uses
Glazing earthenware.
Definition
galena: A mineral form of lead(II)sulphide, PbS, crystallizing in thecubic system; the chief ore of lead. Itusually occurs as grey metallic cubes,frequently in association with silver,arsenic, copper, zinc, and antimony.Important deposits occur in Australia(at Broken Hill), Germany, the USA(especially in Missouri, Kansas, andOklahoma), and the UK.
Reactivity Profile
The reaction between iodine monochloride and any of the following is vigorous: cadmium sulfide, LEAD(II) SULFIDE, silver sulfide, or zinc sulfide [Mellor 2, Supp. 1:502. 1956].
Health Hazard
INHALATION OR INGESTION: Abdominal pain, loss of appetite, weight loss, constipation, apathy or irritability, vomiting, fatigue, headache, weakness metallic taste and muscle incoordination. Lead line on gums. EYES: Irritation. May cause corneal destruction. SKIN: Pain and severe burns.
Fire Hazard
Behavior in Fire: At fire temperatures emits highly toxic and irritating sulfur oxides.
Potential Exposure
Lead sulfide is used in ceramics, infrared radiation detectors, and semiconductors.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention.Note to physician: whole blood lead levels, circulatingplasma/erythrocyte lead concentration ratio, urine ALA,and erythrocyte protoporphyrin fluorescent microscopy mayall be useful in monitoring or assessing lead exposure.Chelating agents, such as edetate disodium calcium (CaEDTA) and penicillamine (not penicillin) are generally useful in the therapy of acute lead intoxication.Antidotes and special procedures for lead: Persons with significant lead poisoning are sometimes treated with CaEDTA while hospitalized. This “chelating” drug causes arush of lead from the body organs into the blood and kidneys, and thus has its own hazards, and must be administered only by highly experienced medical personnel undercontrolled conditions and careful observation. Ca EDTA orsimilar drugs should never be used to prevent poisoningwhile exposure continues or without strict exposure control,as severe kidney damage can result.Note to physician: For severe poisoning BAL [British AntiLewisite, dimercaprol, dithiopropanol (C3H8OS2)] has beenused to treat toxic symptoms of certain heavy metals poisoning. In the case of lead poisoning it may have SOMEvalue. Although BAL is reported to have a large margin ofsafety, caution must be exercised, because toxic effects maybe caused by excessive dosage. Most can be prevented bypremedication with 1-ephedrine sulfate (CAS: 134-72-5).
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Lead sulfide must be stored to avoid contact withoxidizers (such as perchlorates, peroxides, permanganates,chlorates, and nitrates) and chemically active metals (suchas potassium, sodium, magnesium, and zinc), since violentreactions occur. Store in tightly closed containers in a cool,well-ventilated area away from moisture and acids. Lead isregulated by an OSHA Standard 1910.1025. All requirements of the standard must be followed. A regulated,marked area should be established where this chemical ishandled, used, or stored in compliance with OSHAStandard 1910.1045.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required. UN3288 Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels: 6.1- Poisonous materials, Technical Name Required.
Structure and conformation
The space lattice of PbS belongs to the cubic system, and its rock salt structure has a lattice constant of a=0.592 nm and Pb-S=0.296 nm.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, and iodine monochloride. Sulfides react with acids to produce toxic and flammable vapors of hydrogen sulfide.
Lead(II) sulfide Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81138252 +86-18789408387 | 1057@dideu.com | China | 3586 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9641 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6313 | 58 |
| Baoji Guokang Healthchem co.,ltd | +8615604608665 15604608665 | dominicguo@gk-bio.com | CHINA | 9427 | 58 |
Related articles
- The Structure and Uses of Lead(II) sulfide
- Lead(II) sulfide (PbS), also known as galena, is a significant inorganic compound that serves as the principal source of lead ....
- Apr 8,2024
- Is PbS Soluble in water?
- It is a metal sulfide that, like other sulfides in its neighboring spots in the periodic table, is also only sparingly solubl....
- Mar 22,2024
View Lastest Price from Lead(II) sulfide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-04-08 | lead(ii) sulfide
1314-87-0
|
US $80.00 / kg | 1kg | 99% | 100 tons | Hebei Duling International Trade Co. LTD | |
 |
2022-10-11 | Lead(II) sulfide
1314-87-0
|
US $0.00-0.00 / KG | 1KG | 98% | 1ton | Henan Aochuang Chemical Co.,Ltd. | |
 |
2021-08-12 | LEAD(II) SULFIDE
1314-87-0
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- lead(ii) sulfide
1314-87-0
- US $80.00 / kg
- 99%
- Hebei Duling International Trade Co. LTD
-

- Lead(II) sulfide
1314-87-0
- US $0.00-0.00 / KG
- 98%
- Henan Aochuang Chemical Co.,Ltd.
-

- LEAD(II) SULFIDE
1314-87-0
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd