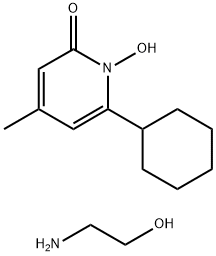
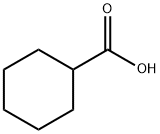
Ciclopirox ethanolamine
- CAS No.
- 41621-49-2
- Chemical Name:
- Ciclopirox ethanolamine
- Synonyms
- CICLOPIROXOLAMINE;Cyclopirox olamine;terit;Ciclobiroxolamine;6-CYCLOHEXYL-1-HYDROXY-4-METHYL-1H-PYRIDIN-2-ONE, 2-AMINO-ETHANOL;6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridinoncompd.with2-aminoethanol;2-aminoethanolcompd.with6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridinone(;lorpox;hoe296;Cicloche
- CBNumber:
- CB0673864
- Molecular Formula:
- C14H24N2O3
- Molecular Weight:
- 268.36
- MDL Number:
- MFCD00078997
- MOL File:
- 41621-49-2.mol
| Melting point | 144 C |
|---|---|
| Density | 0.41g/cm3 at 25℃ |
| vapor pressure | 0-0Pa at 20-50℃ |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| solubility | Sparingly soluble in water, very soluble in ethanol (96 per cent) and in methylene chloride, slightly soluble in ethyl acetate, practically insoluble in cyclohexane. |
| color | White to Off-White |
| LogP | 0.52 |
| CAS DataBase Reference | 41621-49-2(CAS DataBase Reference) |
| FDA UNII | 50MD4SB4AP |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P305+P351+P338 |
| Hazard Codes | Xi,Xn |
| Risk Statements | 36/37/38-42/43-20/22 |
| Safety Statements | 26-36-22 |
| WGK Germany | 2 |
| RTECS | UU7785500 |
| HS Code | 2933790002 |
| Toxicity | LD50 in mice, rats (mg/kg): 2898, 3290 orally (Alpermann, Schutz) |
Ciclopirox ethanolamine price More Price(31)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | C2162700 | Ciclopirox olamine European Pharmacopoeia (EP) Reference Standard | 41621-49-2 | c2162700 | $153 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1134030 | Ciclopirox olamine United States Pharmacopeia (USP) Reference Standard | 41621-49-2 | 125mg | $358 | 2024-03-01 | Buy |
| TCI Chemical | C3545 | Ciclopirox Olamine >98.0%(HPLC)(T) | 41621-49-2 | 1g | $29 | 2024-03-01 | Buy |
| TCI Chemical | C3545 | Ciclopirox Olamine >98.0%(HPLC)(T) | 41621-49-2 | 5g | $87 | 2024-03-01 | Buy |
| Usbiological | 163097 | Ciclopirox Ethanolamine | 41621-49-2 | 1g | $333 | 2021-12-16 | Buy |
Ciclopirox ethanolamine Chemical Properties,Uses,Production
Chemical Properties
White or pale yellow, crystalline powder.
Originator
Batrafen ,Cassella-Riedel, W. Germany ,1980
Uses
Ciclopirox (C432800) derivative. Broad spectrum antimycotic agent with some antibacterial activity.
Uses
Ciclopirox ethanolamine is a broad spectrum antimycotic agent with some antibacterial activity.
Uses
Ciclopirox olamine (Loprox, Penlac) is a synthetic, broad-spectrum hydroxypyridone antifungal agent. It is chemically unrelated to the imidazoles or any other antifungal agent currently available in the United States. It appears to act through intracellular depletion of substrates and/or ions principally by inhibition of transmembrane transport of these substances. It is active against dermatophytes, yeast, and Pityrosporum orbiculare. It also demonstrates activity against Trichomonas vaginalis, some mycoplasma, and some gram-positive and gram-negative bacteria.
Definition
ChEBI: The ethanolamine salt of ciclopirox. A broad spectrum antigfungal agent, it also exhibits antibacterial activity against many Gram-positive and Gram-negative bacteria, and has anti-inflammatory properties. It is used a a topical treatment of fungal skin an nail infections.
Indications
Ciclopirox olamine (Loprox, Penlac) is a synthetic, broad-spectrum hydroxypyridone antifungal agent. It is chemically unrelated to the imidazoles or any other antifungal agent currently available in the United States. It appears to act through intracellular depletion of substrates and/or ions principally by inhibition of transmembrane transport of these substances. It is active against dermatophytes, yeast, and Pityrosporum orbiculare. It also demonstrates activity against Trichomonas vaginalis, some mycoplasma, and some gram-positive and gram-negative bacteria.
Manufacturing Process
Ciclopirox may be produced as follows: 2 g of 4-methyl-6-cyclohexyl-2-pyrone
were heated with 1 g of hydroxylamine hydrochloride and 5 g of 2-
aminopyridine to 80 C for 8 hours.
The reaction mixture was then dissolved in methylene chloride, the amine was
removed by shaking with dilute hydrochloric acid, the reaction product was
extracted from the organic phase by means of dilute sodium hydroxide
solution and the alkaline solution was acidified with acetic acid to a pH value
of 6. The 1-hydroxy-4-cnethyl-6-cyclohexyl-2-pyridone precipitated in
crystalline form. It was filtered off with suction, washed with water and dried.
The yield was 1.05 g (49% of theory); melting point 143 C.
Reaction of ciclopirox with ethanolamine gives the desired prod
brand name
Loprox (Hoechst- Roussel).
Therapeutic Function
Antifungal
Biological Activity
ciclopirox ethanolamine, working as an iron chelator, suppresses a substantial number of clinically relevant dermatophytes, yeasts, and molds, including the frequently azole-resistant candida species candida glabrata, candida krusei, and candida guilliermondii. moreover, ciclopirox has been proved to inhibit a wide range of bacteria in humans, including many gram-positive and gram-negative species pathogenic bacteria. [1]
Clinical Use
6-Cyclohexyl-1-hydroxyl-4-methyl-2(1H)-pyridinoneethanolamine salt (Loprox) is a broad-spectrum antifungalagent intended only for topical use. It is active against dermatophytesas well as pathogenic yeasts (C. albicans) thatare causative agents for superficial fungal infections.
Ciclopirox is considered an agent of choice in the treatmentof cutaneous candidiasis, tinea corporis, tinea cruris,tinea pedis, and tinea versicolor. It is a second-line agent forthe treatment of onychomycosis (ringworm of the nails).Loprox is formulated as a cream and a lotion, each containing1% of the water-soluble ethanolamine salt. Ciclopirox isbelieved to act on cell membranes of susceptible fungi atlow concentrations to block the transport of amino acids intothe cells. At higher concentrations, membrane integrity islost, and cellular constituents leak out.
Clinical Use
Ciclopirox olamine (Loprox) is a pyridone derivative available for the treatment of cutaneous dermatophyte infections, cutaneous C. albicans infections, and tinea versicolor caused by Malassezia furfur. It interferes with fungal growth by inhibiting macromolecule synthesis.
Safety Profile
Poison by intravenous andintraperitoneal routes. Moderately toxic by ingestion andsubcutaneous routes. Experimental reproductive effects.When heated to decomposition it emits toxic fumes ofNOx.
in vitro
ciclopirox inhibited dermatophytes and yeasts pathogenic with mics of 0.98-3.9 μg/ml. studies from c. albicans cells demonstrated that after being rapidly absorbed, ciclopirox largely accumulated intracellular with a concentration of 200 times greater than those in the medium. high concentration of ciclopirox resulted in the loss of folin-positive substances and potassium ions, by this way this agent could lead to cellular leakage without breaking the cell wall. similarly, by decreasing the uptake of precursors of the macromolecules or by decreasing the uptake of essential ions such as potassium ions and phosphate, ciclopirox blocked the synthesis of protein, rna, and dna in growing fungal cells. the chelation of metal ions and the suppression of iron-dependent enzymes were crucial for ciclopirox to exert antifungal effects. ciclopirox alone intensively suppressed the growth of aspergillus fumigatus b5233 conidia. ciclopirox also exhibited synergistic antifungal effect when being combined used with ketoconazole. [1]
in vivo
the effect of ciclopirox on endogenous hif-1 target gene-vegf was investigated using different animal organ models including mouse skin wound model, rat kidney model and chicken chorioallantoic membrane model. according to the results, cpx functionally activated hif-1, induced vegf expression and accelerated angiogenesis. [2]
IC 50
ciclopirox ethanolamine, a broad-spectrum antifungal agent, inhibits dermatophytes and yeasts pathogenic with a minimal inhibitory concentration (mic) of 0.98-3.9 μg/ml.
References
[1]niewerth m, kunze d, seibold m, schaller m, korting hc and hube b. ciclopirox olamine treatment affects the expression pattern of candida albicans genes encoding virulence factors, iron metabolism proteins, and drug resistance factors. antimicrob agents chem. 2003 jun; 47(6): 180517.
[2]linden t, katschinski dr, eckhardt k, scheid a, pagel h and wenger rh. the antimycotic ciclopirox olamine induces hif-1 stability, vegf expression, and angiogenesis. faseb. 2003 feb; 17: 761–3.
[3]subissi a, monti d, togni g and mailland f. recent nonclinical and clinical data relevant to its use as a topical antimycotic agent. drugs. 2010 nov; 70(16): 2133-52.
Ciclopirox ethanolamine Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| APOLLO HEALTHCARE RESOURCES | +6596580999 | sales@apollo-healthcare.com.sg | Singapore | 400 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@airuikechemical.com | China | 994 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15371 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9352 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 | kaia@neputrading.com | China | 1011 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
View Lastest Price from Ciclopirox ethanolamine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-09 | cyclopirox Olamine
41621-49-2
|
US $0.00-0.00 / kg | 1kg | 99.99% | 20 tons | airuikechemical co., ltd. | |
 |
2023-09-06 | Ciclopirox ethanolamine
41621-49-2
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-06-15 | Ciclopirox ethanolamine
41621-49-2
|
US $484.00 / KG | 1KG | 99% GMP | 20T | Baoji Guokang Bio-Technology Co., Ltd. |
-

- cyclopirox Olamine
41621-49-2
- US $0.00-0.00 / kg
- 99.99%
- airuikechemical co., ltd.
-

- Ciclopirox ethanolamine
41621-49-2
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Ciclopirox ethanolamine
41621-49-2
- US $484.00 / KG
- 99% GMP
- Baoji Guokang Bio-Technology Co., Ltd.