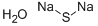Sodium sulfide nonahydrate
- CAS No.
- 1313-84-4
- Chemical Name:
- Sodium sulfide nonahydrate
- Synonyms
- SODIUM SULPHIDE HYDRATE;water treatMent;liuhuajian;Sodium suL;sulfuredesodium;sulfur dyestuff;jiushuiliuhuana;dyeing auxiliary;SodiumSulphideGr;fide nonahydrate
- CBNumber:
- CB1100945
- Molecular Formula:
- H2Na2OS
- Molecular Weight:
- 96.06
- MDL Number:
- MFCD00149183
- MOL File:
- 1313-84-4.mol
- MSDS File:
- SDS
| Melting point | 50 °C |
|---|---|
| Boiling point | 920°C |
| Density | 1.427 |
| storage temp. | 2-8°C |
| solubility | Aqueous Acid (Slightly), Water (Sparingly) |
| form | Solid |
| color | colorless or slightly yellow |
| Specific Gravity | 1.427 |
| Water Solubility | 180 G/L (25 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,8681 |
| Stability | Stable. Hygroscopic. Light-sensitive. Flammable. Avoid exposure to air, acids, oxidizers, strong bases, strong reducing agents, most common metals. |
| InChIKey | GDQBTRYKLOBMLL-UHFFFAOYSA-N |
| CAS DataBase Reference | 1313-84-4(CAS DataBase Reference) |
| FDA UNII | C02T02993U |
| EPA Substance Registry System | Sodium sulfide (Na2S), nonahydrate (1313-84-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS06,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H311-H314-H410 | |||||||||
| Precautionary statements | P260-P273-P280-P301+P312-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | C,N,T | |||||||||
| Risk Statements | 31-34-50-24-22 | |||||||||
| Safety Statements | 26-45-61-36/37/39 | |||||||||
| RIDADR | UN 1849 8/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | WE1925000 | |||||||||
| F | 13 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28301010 | |||||||||
| Toxicity | mouse,LD50,intraperitoneal,53mg/kg (53mg/kg),Comptes Rendus Hebdomadaires des Seances, Academie des Sciences. Vol. 257, Pg. 791, 1963. | |||||||||
| NFPA 704 |
|
Sodium sulfide nonahydrate price More Price(19)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SX0770 | Sodium Sulfide, Nonahydrate meets analytical specifications of USP/NF GR ACS | 1313-84-4 | 500g | $128 | 2024-03-01 | Buy |
| Sigma-Aldrich | 208043 | Sodium sulfide nonahydrate ACS reagent, ≥98.0% | 1313-84-4 | 5g | $36.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 208043 | Sodium sulfide nonahydrate ACS reagent, ≥98.0% | 1313-84-4 | 100g | $69.6 | 2024-03-01 | Buy |
| Alfa Aesar | 036622 | Sodium sulfide nonahydrate, ACS 98.0% min | 1313-84-4 | 250g | $61.6 | 2023-06-20 | Buy |
| Alfa Aesar | 036622 | Sodium sulfide nonahydrate, ACS 98.0% min | 1313-84-4 | 1kg | $145 | 2023-06-20 | Buy |
Sodium sulfide nonahydrate Chemical Properties,Uses,Production
Physical Properties
The nonahydrate is a yellowish-white crystalline solid; tetragonal crystals; odor of hydrogen sulfide; the color changes on exposure to light and air, first turning to yellow and then becoming brownish-black, deliquescent; density 1.43 g/cm3; decomposes at about 50°C; very soluble in water; aqueous solution strongly alkaline; slightly soluble in alcohol; insoluble in ether.
Uses
Sodium sulfide is used in making sulfur dyes; for dehairing of hides; removing sulfur from viscous rayon; engraving and lithography; cotton printing; manufacturing rubber; paper pulp; and as a photographic reagent. Other major applications are for treating paper and for extracting gold ores where oxidized metal ores are converted to sulfides prior to froth flotation. Sodium sulfide also is used in preparing many other sulfides and as an analytical reagent.
Preparation
Sodium sulfide is prepared by heating sodium bisulfate with sodium chloride and coal above 950°C. The product mixture is extracted with water and the hydrated sulfide is obtained from the solution by crystallization:
NaHSO4 + NaCl + 2C → Na2S + 2CO2↑ + HCl↑
Sodium sulfide also is produced from its elements in liquid ammonia:
Na + 2S → Na2S
Reactions
Sodium sulfide in solid form reacts with carbon dioxide in the presence of moisture to form hydrogen sulfide and sodium carbonate. Thus, the H2S odor of sodium sulfide crystals is attributed to its exposure to moist air:
Na2S + H2O + CO2 → Na2CO3 + H2S
In aqueous solution, sodium sulfide reacts with a number of metal salts forming insoluble sulfides.
When added to dilute mineral acids, hydrogen sulfide is generated.
Chemical Properties
colourless, white or yellow crystals with an odour of rotten eggs
Uses
It is the precursor material for preparation of cadmium sulfide quantum dots. Also employed in the pulp and paper industry. It is likewise employed in the making of colors and dyestuffs. It is too useful in the trace quantities for the rapid synthesis of small silver nanocubes through mediating polyol reduction. It has as a significant role in breaking up the feather keratin useful for the preparation of bio-polymer.
Uses
Sodium sulfide nonahydrate has been used as a source of H2S, to study the effect of H2S on amyloid fibrils associated with neurodegenerative diseases. It has been used in the preparation of sulfide standards for the quantitative analysis of sulfides in raw wastewater (sewage) samples.
It serves as a catalyst during synthesis of thioamides.
Uses
sodium sulphide nonahydrate has the following uses:sulfur dyestuff; leather depilatory;rayon denitration;dyeing auxiliary;sodium sulphide;including crystallization water; sodium sulphide or sulfureT;dyeing auxiliary.
General Description
Sodium sulfide nonahydrate participates in the conversion of nitroxides to amines, via reduction.
Purification Methods
Some purification of the hydrated salt can be achieved by selecting large crystals and removing the surface layer (contaminated with oxidation products) by washing with distilled water. Other metal ions can be removed from Na2S solutions by passage through a column of Dowex ion-exchange A-1 resin, Na+-form. The hydrated salt can be rendered anhydrous by heating it in a stream of H2 or N2 until water is no longer evolved. (The resulting cake should not be heated to fusion because it is readily oxidised.) Recrystallise it from distilled water [Anderson & Azowlay J Chem Soc, Dalton Trans 469 1986]. Note that sodium sulfide hydrolyses in H2O to form NaHS + H2O, and is therefore alkaline. A 0.1N solution in H2O is 86% hydrolysed at room temperature. Its solubility in H2O is 8% at 0o, 12% at 20o and 30% at 50o. The anhydrous salt is obtained by allowing it to stand in a vacuum over conc H2SO4 or P2O5 at 45o to start with, then at 30-35o when the salt contains 4% of water. The last traces of water are removed by heating to 700o in a glass or porcelain tube in a stream of H2 to give pure H2S. [Fehér in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 358-360 1963.]
Sodium sulfide nonahydrate Preparation Products And Raw materials
Raw materials
Preparation Products
1of6
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
View Lastest Price from Sodium sulfide nonahydrate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-01-31 | Sodium Sulfide Nonahydrate
1313-84-4
|
US $21.00 / Kg/Bag | 25KG | 99.99% | 200ton | Hebei Mojin Biotechnology Co., Ltd | |
 |
2021-09-03 | Sodium sulfide nonahydrate
1313-84-4
|
US $10.00 / KG | 1KG | 99% | 10 mt | Hebei Guanlang Biotechnology Co., Ltd. | |
 |
2021-08-12 | SODIUM SULPHIDE HYDRATE
1313-84-4
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- Sodium Sulfide Nonahydrate
1313-84-4
- US $21.00 / Kg/Bag
- 99.99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Sodium sulfide nonahydrate
1313-84-4
- US $10.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
-

- SODIUM SULPHIDE HYDRATE
1313-84-4
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd