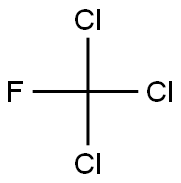
Trichlorofluoromethane
- CAS No.
- 75-69-4
- Chemical Name:
- Trichlorofluoromethane
- Synonyms
- CFCl3;CFC-11;CCl3F;TRICHLOROMONOFLUOROMETHANE;f11;f-11;Trichlorfluormethan;FLUOROTRICHLOROMETHANE;Methane, trichlorofluoro-;f11b
- CBNumber:
- CB1127379
- Molecular Formula:
- CCl3F
Lewis structure
- Molecular Weight:
- 137.37
- MDL Number:
- MFCD00000784
- MOL File:
- 75-69-4.mol
- MSDS File:
- SDS
| Melting point | -111°C |
|---|---|
| Boiling point | 23.8°C |
| Density | 1.494 |
| vapor density | 5.04 (vs air) |
| vapor pressure | 12.85 psi ( 20 °C) &_& 39.17 psi ( 55 °C) |
| refractive index | 1.382 |
| Flash point | 2 °C |
| storage temp. | 2-8°C |
| solubility | water: soluble1g/L |
| color | Colorless, odorless liquid |
| Odor | Odorless; weak chlorinated solvent. |
| Water Solubility | insoluble. 0.124 g/100 mL |
| Merck | 13,9714 |
| BRN | 1732469 |
| Henry's Law Constant | At 25 °C: 88.2 and 123 in distilled water and seawater, respectively (Hunter-Smith et al., 1983) |
| Exposure limits | NIOSH REL: ceiling 1,000 ppm (5,600 mg/m3), IDLH 2,000 ppm; OSHA PEL: TWA 1,000 ppm; ACGIH TLV: ceiling 1,000 ppm (adopted). |
| Dielectric constant | 3.1(21℃) |
| Stability | Stable. Incompatible with bronze, silver, copper, beryllium, alkali and alkaline earth metals. |
| InChIKey | CYRMSUTZVYGINF-UHFFFAOYSA-N |
| CAS DataBase Reference | 75-69-4(CAS DataBase Reference) |
| FDA 21 CFR | 701.30 |
| EWG's Food Scores | 1 |
| FDA UNII | 990TYB331R |
| NIST Chemistry Reference | Trichloromonofluoromethane(75-69-4) |
| EPA Substance Registry System | CFC-11 (75-69-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H315-H319-H331-H336-H351-H361d-H372-H420 | |||||||||
| Precautionary statements | P301+P312-P302+P352-P304+P340+P311-P305+P351+P338-P308+P313-P502 | |||||||||
| Hazard Codes | Xn;N,Xi,N,Xn,T,F | |||||||||
| Risk Statements | 20-59-23-21/22-36/37/38-20/22-11-23/25-39/23/24/25-23/24/25-21-67-66-36 | |||||||||
| Safety Statements | 23-24/25-59-61-45-36/37-36-26-16-24-7 | |||||||||
| RIDADR | 3082 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | PB6125000 | |||||||||
| Hazard Note | Irritant | |||||||||
| Toxicity | Drinking water standard: No MCLGs or MCLs have been proposed although trichlorofluoromethane has been listed for regulation (U.S. EPA, 1996). A DWEL of 100 μg/L was recommended (U.S. EPA, 2000). | |||||||||
| IDLA | 2,000 ppm | |||||||||
| NFPA 704 |
|
Trichlorofluoromethane Chemical Properties,Uses,Production
Description
Trichlorofluoromethane is a chlorofluorocarbon (CFC) which is commonly used as a refrigerant, a foaming or blowing agent in industry, a solvent, an aerosol propellant, and in chemical syntheses.
Trichlorofluoromethane is a colorless, odorless gas at normal temperatures and pressures. Under high pressures as in cans, tanks or refrigerators it is in liquid form. When released from a pressurized container it evaporates almost instantly and can cause freezing at the point of release. At very high concentrations in air it may smell like ether. It is slightly soluble in water but evaporates quickly if exposed to air. The chemical formula for Trichlorofluoromethane is CFCL3.
Chemical Properties
Fluorotrichloromethane is a colorless liquid or gas. Chlorinated solvent odor. The Odor Threshold is 5.0 ppm.
Occurrence
Trichlorofluoromethane is man-made and its presence in the environment is due to releases from common household and industrial uses. It is extremely stable in the atmosphere and does not degrade naturally. It can also be produced as an industrial by-product wherever chlorine products are used; including small quantities which are formed by reaction of chlorine disinfectants with organic pollutants in water. It is released into the air by leaking refrigeration units and air conditioners and by spray paint, spray varnish, spray cosmetics and other sprays in which it has been used as a propellant. Since 1978 many uses of trichlorofluoromethane as a propellant have been prohibited by law in the U.S. It can still be used as a propellant in some specialized products used in businesses and industries. It may also be found in air emissions and waste waters from a number of industries particularly refrigeration, electronics and foam manufacturing.
Uses
Trichlorofluoromethane is a coolant in conditioning systems. It is a bulking agent for polymer foams. It is also used as a degrease solvent for printed circuit boards and to dry clean textiles. It is used as an aerosol propellant in various industries.
Uses
Aerosol propellant; refrigerant and blowing agent; solvent for cleaning and degreasing.
Uses
In refrigeration machinery requiring a refrigerant effective at negative pressures. As aerosol propellant.
Definition
ChEBI: A one-carbon compound that is methane in which the hydrogens have been replaced by three chlorine and one fluorine atom.
General Description
A clear light colored liquid. Nearly odorless. Denser than water. Poses low acute health hazard to humans. Primary hazard is to the environment. Immediate steps should be taken to limit spread to the environment. Easily penetrates the soil to contaminate groundwater and nearby waterways.
Air & Water Reactions
Water soluble. Hydrolyzed slowly.
Reactivity Profile
Trichlorofluoromethane is incompatible with alkali or alkaline earth metals, powdered aluminum, zinc and beryllium. Trichlorofluoromethane reacts violently with barium and lithium.
Health Hazard
Breathing concentrations approaching 10% in air will cause dizziness and drowsiness. Contact with tissues may cause frostbite.
Fire Hazard
Special Hazards of Combustion Products: Produces irritating and toxic products when heated to decomposition temperatures.
Chemical Reactivity
Reactivity with Water: No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
High concentrations cause narcosis and anesthesia in humans. Human systemic effects by inhalation: conjunctiva irritation, fibrosing alveolitis, and liver changes. Experimental poison by inhalation. Moderately toxic by intraDeritoneal route. Reacts violentlv with I aluminum, barium, or lithium. When heated to decomposition it emits highly toxic fumes of Fand Cl-. Used as an aerosol propellant, refrigerant, and blowing agent for polymeric foams. See also CHLORINATED HYDROCARBONS, ALIPHATIC; and FLUORIDES.
Potential Exposure
This material is used as a refrigerant; aerosol propellant; and foaming agent; as blowing agent in production of polyurethane foams.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Carcinogenicity
When administered by gavage to groups of 50 male and 50 female mice at daily doses of 1962 or 3952mg/kg, 5 days/week for 78 weeks followed by a 13week observation period, there was no evidence of carcinogenicity. Also,when given to rats at daily doses of 488 and 977mg/kg for males and 538 and 1077mg/kg for females, again for 5 days/week for 78 weeks, but followed by a 28–33 week observation period, there was no evidence of carcinogenicity. Maltoni et al. exposed groups of 90 male and 90 female Sprague–Dawley rats and groups of 60 male and 60 female Swiss mice by inhalation to levels of 1000 and 5000 ppm 4 h/day, 5 days/week for life. The exposures did not produce evidence of carcinogenicity.
Environmental Fate
Biological. In a static-culture-flask screening test, trichlorofluoromethane was statically
incubated in the dark at 25 °C with yeast extract and settled domestic wastewater inoculum. No
significant degradation was observed after 28 d of incubation. At substrate concentrations of 5 and
10 mg/L, percent losses due to volatilization were 58 and 37% after 10 d (Tabak et al., 1981).
Chemical/Physical. When trichlorofluoromethane (50 μg/L) in an ultrasonicator was exposed to
20-kHz ultrasound at 5 °C, nearly 100% degradation was achieved after 6 min. During sonication,
the pH of the aqueous solution decreased, which is consistent with the formation of HCl,
hydrofluoric acid, and acidic species from fluorine and chlorine. In this experiment <5% of
trichlorofluoroethane was lost to volatilization (Cheung and Kurup, 1994).
storage
Color Code—Green: General storage may be used.Prior to working with this chemical you should be trainedon its proper handling and storage. Trichlorofluoromethanemust be stored to avoid contact with chemically activemetals, such as aluminum or lithium, since violent reactionsoccur. Store in tightly closed containers in a cool, well-ventilated area away from sources of heat.
Shipping
Hazard Class: 2.2; Labels: 2.2-Nonflammable compressed gas, Technical Name Required.
Incompatibilities
Chemically active and powdered metals: aluminum, barium, sodium, potassium, calcium, powdered aluminum; zinc, magnesium.
Waste Disposal
Incineration, preferably after mixing with another combustible fuel. Care must be exer cised to assure complete combustion to prevent the forma tion of phosgene. An acid scrubber is necessary to remove the halo acids produced. Consult with environmental regu latory agencies for guidance on acceptable disposal prac tices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations govern ing storage, transportation, treatment, and waste disposal.
Trichlorofluoromethane Preparation Products And Raw materials
75-69-4(Trichlorofluoromethane)Related Search:
1of4