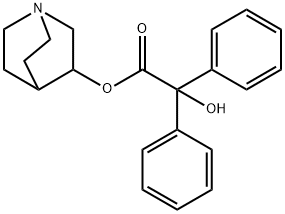
3-Quinuclidinyl benzilate
- CAS No.
- 6581-06-2
- Chemical Name:
- 3-Quinuclidinyl benzilate
- Synonyms
- BZ;cs4030;ea2277;CS 4030;EA 2277;Ro 2-330;ro2-3308;RO 2-3308;NSC 173698;3-Chinuclidylbenzilate
- CBNumber:
- CB1244838
- Molecular Formula:
- C21H23NO3
- Molecular Weight:
- 337.42
- MDL Number:
- MFCD01658356
- MOL File:
- 6581-06-2.mol
| Melting point | 164.5°C |
|---|---|
| Boiling point | 473.72°C (rough estimate) |
| Density | 1.1644 (rough estimate) |
| refractive index | 1.5614 (estimate) |
| storage temp. | 2-8°C |
| solubility | chloroform: but decomposes within 24 hrssoluble |
| pka | 11.28±0.29(Predicted) |
| form | powder |
| color | white |
| CAS DataBase Reference | 6581-06-2 |
| EWG's Food Scores | 1 |
| FDA UNII | E69DLR7470 |
| NIST Chemistry Reference | 3-Quinuclidinyl benzilate(6581-06-2) |
| EPA Substance Registry System | Benzeneacetic acid, .alpha.-hydroxy-.alpha.-phenyl-, 1-azabicyclo[2.2.2]oct-3-yl ester (6581-06-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H300 |
| Precautionary statements | P264-P301+P310 |
| Hazard Codes | Xn,T+ |
| Risk Statements | 22-26 |
| Safety Statements | 28-36/37-45 |
| WGK Germany | 3 |
| RTECS | DD4638000 |
| HS Code | 29333910 |
| Toxicity | LD50 intraperitoneal in mouse: 103mg/kg |
3-Quinuclidinyl benzilate Chemical Properties,Uses,Production
Description
The 3-quinuclidinyl benzilate (3-QNB) was first discovered by Hoffmann-La Roche in 1951 during research investigation that involved development of antispasmodic agents, resembling atropine, for treating gastrointestinal conditions. Soon after, the US Army investigated 3-QNB under the name of EA2277 agent and was standardized for use in chemical munitions in 1961 under the name agent of BZ. The United States declared complete destruction of its stockpile of BZ by 1989. Moreover, Agent 15 is speculated either to be identical to BZ or a closely related derivative, and has similar physicochemical properties as BZ. In 1998, Agent 15 was speculated to be stockpiled by Iraq but later on intelligence reports did not find any conclusive evidence for such claim1. The BZ is a glycolate anticholinergic chemical related to atropine, scopolamine, and, hyoscyamine. It is odorless, nonirritating, and is stable in most solvents. It has a half-life of 3–4 weeks in moist air and is extremely persistent in soil, water, and most surfaces. BZ has a slow onset and a long duration-of-action. The rate of BZ action climaxed after 9.5 h of exposure and the duration-of-action could last up to 96 h. Moreover, most of the causalities of BZ exposure will require physical restraint and continuous monitoring to prevent self-injury and paranoia symptoms during recovery2. Recent speculation about the usage of Agent 15 in Syrian civil war had been reported but no confirmation findings had been established3.
Chemical Properties
Colourless solid
Uses
3-quinuclidinyl benzilate(BZ) is a nonlethal chemical warfare agent that following a delayed onset is incapacitating and causes severe hallucinations. US Army intended to use BZ in critical point situations such as special operation, incapacitating adversaries during raid, and overcoming fortified field positions. Medically, BZ is used as a muscarinic receptor antagonist.
General Description
Colorless liquid, odorless to fruity.
Environmental Fate
BZ acts by blocking the action of acetylcholine on the central
and peripheral nervous systems. It is a tertiary amine and
crosses the blood–brain barrier. BZ on acute exposure
increases both heart and respiratory rates, dilates the pupils,
and causes paralysis of the eye muscles necessary for near
focusing. It also causes dry mouth and skin, elevates body
temperature, impairs coordination, and causes flushing of the
skin, hallucinations, stupor, forgetfulness, and confusion.Within 15 min to 4 h following exposure, the principal effects
are dizziness, involuntary muscle movements, near vision
difficulty, and total incapacitation. From 6 to 10 h after
exposure, the effects are psychotropic and full recovery is expected after 4 days.
The peripheral nervous system effects are considered as
understimulation of the end organs. This decreased stimulation
of eccrine and apocrine sweat glands in the skin results in dry
skin and a dry mouth, and is considered ‘dry as a bone.’ The
reduction in the ability to dispel heat by evaporative cooling
decreases sweating, and the compensatory cutaneous vasodilation
causes the skin to become warm or ‘hot as a hare’ and ‘red
as a beet.’ This is similar to the atropine flush. The decreased
heat loss also results in an increased core temperature.
Toxicity evaluation
QNB-BZ or Agent 15 has been tested on different surfaces by the US Army Medical Research Institute of Chemical Defense and the overall conclusion was that QNB-BZ persists as a parent molecule for an extended period of time reaching 72 h4.
3-Quinuclidinyl benzilate Preparation Products And Raw materials
Raw materials
Preparation Products
Related articles
- Toxicity of 3-Quinuclidinyl benzilate
- 3-Quinuclidinyl benzilate (QNB) — IUPAC name 1-azabicyclo[2.2.2]octan-3-yl hydroxy(diphenyl)acetate; US Army code EA-2277; NAT....
- Dec 3,2021
6581-06-2(3-Quinuclidinyl benzilate)Related Search:
1of4