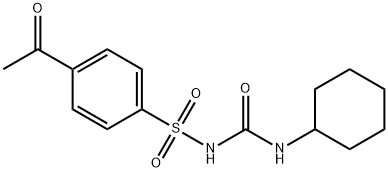
ACETOHEXAMIDE
- CAS No.
- 968-81-0
- Chemical Name:
- ACETOHEXAMIDE
- Synonyms
- dymelor;Cyclamide;u14812;u-14812;dimelin;dimelor;minoral;ordimel;tsiklamid;DiMelor-d6
- CBNumber:
- CB1316029
- Molecular Formula:
- C15H20N2O4S
- Molecular Weight:
- 324.4
- MDL Number:
- MFCD00072156
- MOL File:
- 968-81-0.mol
| Melting point | 188-190° (GB 912789); mp 175-177° (Marshall) |
|---|---|
| Density | 1.2528 (rough estimate) |
| refractive index | 1.6930 (estimate) |
| storage temp. | Refrigerator |
| solubility | DMSO: ~45 mg/mL |
| form | solid |
| pka | 4.32±0.10(Predicted) |
| color | white |
| Water Solubility | 0.25g/L(25 ºC) |
| CAS DataBase Reference | 968-81-0 |
| FDA UNII | QGC8W08I6I |
| NCI Drug Dictionary | acetohexamide |
| ATC code | A10BB31 |
| EPA Substance Registry System | Acetohexamide (968-81-0) |
SAFETY
Risk and Safety Statements
| Safety Statements | 36 |
|---|---|
| WGK Germany | 2 |
| RTECS | YR7350000 |
| Toxicity | LD50 oral in rat: > 2gm/kg |
ACETOHEXAMIDE price More Price(17)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | A178 | Acetohexamide analytical standard | 968-81-0 | 1g | $87.6 | 2022-05-15 | Buy |
| Cayman Chemical | 23900 | Acetohexamide ≥95% | 968-81-0 | 50mg | $32 | 2024-03-01 | Buy |
| Cayman Chemical | 23900 | Acetohexamide ≥95% | 968-81-0 | 100mg | $61 | 2024-03-01 | Buy |
| TRC | A162650 | Acetohexamide | 968-81-0 | 100mg | $55 | 2021-12-16 | Buy |
| TRC | A162652 | Acetohexamide-d11 | 968-81-0 | 1mg | $150 | 2021-12-16 | Buy |
ACETOHEXAMIDE Chemical Properties,Uses,Production
Originator
Dymelor ,Lilly ,US ,1964
Uses
antifungal
Uses
Labelled Acetohexamide, a sulfonylurea derivative. Acetohexamide is a hyopglycemic agent with moderate uricosuric activity. Acetohexamide is a first generation medication used in the treatment of diab etes metilus type 2.
Uses
Acetohexamide is a sulfonylurea derivative. Acetohexamide is a hyopglycemic agent with moderate uricosuric activity. Acetohexamide is a first generation medication used in the treatment of diabetes metilus type 2.
Definition
ChEBI: An N-sulfonylurea that is urea in which a hydrogen attached to one of the nitrogens is replaced by a p-acetylphenylsulfonyl group, while a hydrogen attached to the other nitrogen is replaced by a cyclohexyl group.
Manufacturing Process
Preparation of p-Acetylbenzenesulfonamide: 100 grams of paminoacetophenone
were dissolved in a solvent mixture containing 165 ml of
12 N hydrochloric acid and 165 ml of glacial acetic acid. The mixture was
cooled with stirring to about 0°C. A solution containing 56.2 grams of sodium
nitrite and 175 ml of water was added dropwise with stirring to the acidic
solution while maintaining the temperature below 5°C.
After the addition had been completed, the acidic solution containing pacetylphenyldiazonium
chloride formed in the above reaction was added
dropwise with stirring to a mixture of 530 ml of glacial acetic acid and 530 ml
of benzene which had been previously cooled, and the cooled solution
saturated with sulfur dioxide and to which had been added 34 g of cupric
chloride dihydrate. After the addition had been completed, the reaction
mixture was stirred at about 40°C for three hours, and was then poured into
3,000 ml of an ice-water mixture.
The benzene layer containing p-acetylbenzenesulfonyl chloride formed in the
above reaction was separated, and the acidic aqueous phase was extracted
twice with 250 ml portions of benzene. The benzene layers were combined,
the combined extracts were filtered, and the benzene was evaporated from
the resulting filtrate in vacuum.
The solid residue comprising p-acetylbenzenesulfonyl chloride was dissolved in
100 ml of dioxane, and the solution was added to 200 ml of 14% aqueous
ammonium hydroxide. The resulting solution was stirred overnight at ambient
room temperature. The p-acetylbenzenesulfonamide thus prepared was
collected by filtration. Recrystallization of the filter cake from aqueous ethanol
yielded purified p-acetylbenzenesulfonamide melting at about 176°C to 179°C.
Preparation of N-p-Acetylphenylsulfonyl-N'-Cyclohexylurea: A reaction mixture
consisting of 32.7 grams of p-acetylbenzenesulfonamide and 64 grams of
anhydrous potassium carbonate in 350 ml of anhydrous acetone was stirred at
refluxing temperature for about 1% hours, thus forming the potassium salt of
p-acetylbenzenesulfonamide. 30.9 grams of cyclohexylisocyanate were added
dropwise to the reaction mixture. Refluxing and stirring were continued during
the course of the addition and for an additional 16 hours.
The acetone was removed by evaporation in vacuum, and about 750 ml of
water were added to dissolve the resulting residue. The solution was filtered.
The potassium salt of N-p-acetylphenylsulfonyl-N'-cyclohexylurea formed in
the above reaction, being water-soluble, passed into the filtrate. Acidification
of the filtrate with 6 N aqueous hydrochloric acid caused the precipitation of
N-p-acetylphenylsulfonyl-N'-cyclohexylurea which was collected by filtration.
Recrystallization of the filter cake from 90% aqueous ethanol yielded purified
N-p-acetylphenylsulfonyl-N'-cyclohexylurea melting at about 188-190°C.
brand name
Dymelor (Lilly).
Therapeutic Function
Hypoglycemic
General Description
Acetohexamide is 4-acetyl-N-[(cyclohexylamino)carbonyl]benzenesulfonamide; or 1-[(p-acetylphenyl)sulfonyl]-3-cyclohexylurea; or 1-(p-acetylbenzenesulfonyl)-3-cyclohexylurea(generic). Acetohexamide incorporates the nearly optimal(for potency) cyclohexyl moiety in the “right-hand” sideof its molecular structure, but a p-acetyl substituent on the“left-side” benzene ring that decreases lipophilicity and israpidly biotransformed by reduction to an active metabolitethat is cleared relatively rapidly (see preceding discussion) independentlyof any P450s.
General Description
White fluffy crystalline powder with almost no odor.
General Description
Acetohexamide, 1-[(p-acetylphenyl)sulfonyl]-3-cyclohexylurea (Dymelor), is relatedchemically and pharmacologically to tolbutamide and chlorpropamide.Like the other sulfonylureas, acetohexamidelowers the blood sugar level, primarily by stimulating the releaseof endogenous insulin.
Air & Water Reactions
Water insoluble.
Reactivity Profile
An amide. Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Fire Hazard
Flash point data for ACETOHEXAMIDE are not available; however, ACETOHEXAMIDE is probably combustible.
Clinical Use
Acetohexamide is metabolized in the liver to a reducedform, the α -hydroxyethyl derivative. This metabolite, themain one in humans, possesses hypoglycemic activity.Acetohexamide is intermediate between tolbutamide andchlorpropamide in potency and duration of effect on bloodsugar levels.
Safety Profile
Human reproductive effects by an unspecified route: stillbirth. Mildly toxic by ingestion. When heated to decomposition it emits very toxic fumes of SO, and NOx,.
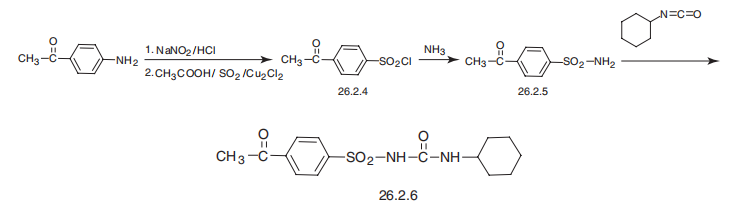
Synthesis
Acetohexamide, 1-(p-acetyl phenylsulfonyl)-3-cyclohexylurea (26.2.6), is made in an analogous scheme by reacting p-chlorobenzenesulfonylamide with cyclohexylisocyanate. The necessary p-acetylbenzenesulfonylamide is made by diazotating of p-aminoacetophenone in the presence of sulfur dioxide and copper(II) chloride, forming the sulfonylchloride 26.2.4, which is reacted further with ammonia to give the sulfonamide (26.2.5). Reacting this with cyclohexylisocyanate gives acetohexamide (26.2.6).
ACETOHEXAMIDE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81148696 +8615536356810 | 1047@dideu.com | China | 3679 | 58 |
| Tianjin Xinshengjiahe Science & Technology Development Co,.Ltd | +86-86-22-87899925 +86-8618522618860 | 18522618860@163.com | China | 694 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9553 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29474 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
| Labnetwork lnc. | +86-27-50766799 +8618062016861 | contact@labnetwork.com | China | 19994 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9309 | 58 |
View Lastest Price from ACETOHEXAMIDE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-06-08 | ACETOHEXAMIDE USP/EP/BP
968-81-0
|
US $1.10 / g | 1g | 99.9% | 100 Tons Min | Dideu Industries Group Limited | |
 |
2020-07-09 | ACETOHEXAMIDE
968-81-0
|
US $3400.00 / KG | 1KG | 98.0% | 1T | Shaanxi Dideu Medichem Co. Ltd | |
 |
2019-12-26 | ACETOHEXAMIDE
968-81-0
|
US $1.00 / g | 1g | 99% | 200kg | Career Henan Chemical Co |
-

- ACETOHEXAMIDE USP/EP/BP
968-81-0
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited
-

- ACETOHEXAMIDE
968-81-0
- US $3400.00 / KG
- 98.0%
- Shaanxi Dideu Medichem Co. Ltd
-

- ACETOHEXAMIDE
968-81-0
- US $1.00 / g
- 99%
- Career Henan Chemical Co