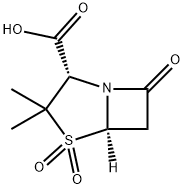
Sulbactam
- CAS No.
- 68373-14-8
- Chemical Name:
- Sulbactam
- Synonyms
- SULBACTAM ACID;sulbactum;CP-45899;penicillanic acid 1,1-dioxide;(2S,5R)-3,3-Dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide;Betamaze;SULBACTAM;Shu ba acid;Sulbactam CRS;Sulbactam >
- CBNumber:
- CB1419201
- Molecular Formula:
- C8H11NO5S
- Molecular Weight:
- 233.24
- MDL Number:
- MFCD00867005
- MOL File:
- 68373-14-8.mol
| Melting point | 154-157℃ |
|---|---|
| alpha | D20 +251° (c = 0.01 in pH 5.0 buffer) |
| Boiling point | 567.7±50.0 °C(Predicted) |
| Density | 1.62±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | H2O: ≥18mg/mL |
| form | lyophilized powder |
| pka | 2.62±0.40(Predicted) |
| color | white to tan |
| optical activity | [α]/D ≥+225°, c = 1 in H2O |
| Water Solubility | Soluble in water at 18mg/ml |
| Merck | 14,8889 |
| Stability | Hygroscopic |
| InChIKey | FKENQMMABCRJMK-RITPCOANSA-N |
| CAS DataBase Reference | 68373-14-8 |
| FDA UNII | S4TF6I2330 |
| ATC code | J01CG01 |
Sulbactam price More Price(49)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHR2576 | Sulbactam Pharmaceutical Secondary Standard; Certified Reference Material | 68373-14-8 | 500MG | $158 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1623670 | Sulbactam United States Pharmacopeia (USP) Reference Standard | 68373-14-8 | 250mg | $436 | 2024-03-01 | Buy |
| Sigma-Aldrich | 94876 | Sulbactam analytical standard | 68373-14-8 | 10mg | $144 | 2022-05-15 | Buy |
| TCI Chemical | S0868 | Sulbactam >98.0%(HPLC)(T) | 68373-14-8 | 5g | $112 | 2024-03-01 | Buy |
| TCI Chemical | S0868 | Sulbactam >98.0%(HPLC)(T) | 68373-14-8 | 25g | $378 | 2024-03-01 | Buy |
Sulbactam Chemical Properties,Uses,Production
Description
Sulbactam is prepared by partial chemical synthesis from penicillins. The oxidation of the sulfur atom to a sulfone greatly enhances the potency of sulbactam. The combination of sulbactam and ampicillin (Unasyn) is now clinically popular.
Originator
Sulbactam-Sodium,Antibiotic Co.,Bulgaria
Uses
A β-lactamase inhibitor.
Uses
antidepressant, dopamine uptake inhibitor
Uses
Sulbactam sodium is a semi-synthetic penem antibiotic formed by the oxidation of penicillanic acid to its sulfone and was invented by Barth at Pfizer in 1978. Sulbactam sodium is a weak antibiotic but its action as an irreversible inhibitor of β-lactamase is exploited to block the degradation of other penicillin derivatives. Sulbactam acts as a synergist with cephalosporins and penicillins against Gram positive bacteria and is used commercially in combination with ampicillin.
Definition
ChEBI: Sulbactam is a member of penicillanic acids.
Manufacturing Process
Sulbactam sodium is semi-synthetic antibiotic of penicillinic group. Start
material for it's synthesis is 6-aminopenicillanic acid. First 6-aminopenicillanic
acid was isolated in 1957 year from benzylpenicilline as resalt of treating of it
by penicillinaze. Benzylpenicilline is produced by microorganism of genus
Streptomyces.
Further, 6-aminopenicillanic acid reacted with bromine, hydrochloric acid and
NaNO2. As a result the 6,6-dibromopenicillanic acid was obtained.
6,6-Dibromopenicillanic acid was oxidized by KMnO4, to give 6,6-dibromo-1,1-The 6,6-dibromo-1,1-dioxopenicillanic acid in presence of Fe was converted to
the 1,1-dioxopenicillanic acid (sulbactam acid). The sulbactam acid was
treated by sodium 2-ethylhexanoate and crude sulbactam sodium was
obtained.
Therapeutic Function
Beta-lactamase inhibitor
Antimicrobial activity
Sulbactam has very weak antimicrobial activity against most bacteria. Its only notable activity is against N. gonorrhoeae, N. meningitidis and Acinetobacter baumannii.
Clinical Use
Sulbactam is penicillanic acid sulfone or 1,1-dioxopenicillanicacid. This synthetic penicillin derivative is a potent inhibitorof S. aureus β-lactamase as well as many β-lactamaseselaborated by Gram-negative bacilli. Sulbactam has weak intrinsicantibacterial activity but potentiates the activity ofampicillin and carbenicillin against β-lactamase–producingS. aureus and members of the Enterobacteriaceae family. Itdoes not, however, synergize with either carbenicillin or ticarcillinagainst P. aeruginosa strains resistant to these agents.Failure of sulbactam to penetrate the cell envelope is a possibleexplanation for the lack of synergy.
Fixed-dose combinations of ampicillin sodium and sulbactamsodium, marketed under the trade name Unasyn assterile powders for injection, have been approved for use inthe United States. These combinations are recommended forthe treatment of skin, tissue, intra-abdominal, and gynecologicalinfections caused by β-lactamase–producing strainsof S. aureus, E. coli, Klebsiella spp., P. mirabilis, B. fragilis,and Enterobacter and Acinetobacter spp.
Sulbactam Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Nanjing Sinoda Biological Technology Co., Ltd | +8613401983379 | sales@njmcn.cn | CHINA | 74 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7786 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@airuikechemical.com | China | 994 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ | sales03@shyrchem.com | CHINA | 738 | 60 |
Related articles
- Sulbactam: Antimicrobial Activity, Susceptibility, Administration and Dosage, Clinical Uses etc.
- Sulbactam sodium is a semisynthetic beta-lactamase inhibitor obtained by oxidation of the thiazolidine sulfur of penicillanic ....
- Mar 18,2022
View Lastest Price from Sulbactam manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-03-29 | Sulbactam
68373-14-8
|
US $8.00-1.00 / kg | 1kg | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2024-03-29 | sulbactam
68373-14-8
|
US $0.00-0.00 / Kg | 1Kg | 99.9% | 200tons | airuikechemical co., ltd. | |
 |
2023-11-20 | Sulbactam
68373-14-8
|
US $0.00 / kg | 1kg | 0.99 | 20 tons | Hebei Yanxi Chemical Co., Ltd. |