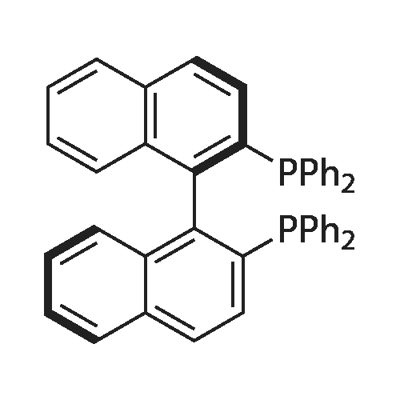
(R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl
- CAS No.
- 76189-55-4
- Chemical Name:
- (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl
- Synonyms
- BINAP;RAC-BINAP;(R)-BINAP;RACEMIC-2,2'-BIS(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHYL;(+)-BINAP;(+/-)-BINAP;98% (R)-BINAP;(R)-(+)-BINAP;RACEMIC-BINAP;(R)-BINAP,99%e.e.
- CBNumber:
- CB2186988
- Molecular Formula:
- C44H32P2
- Molecular Weight:
- 622.69
- MDL Number:
- MFCD00010805
- MOL File:
- 76189-55-4.mol
- MSDS File:
- SDS
| Melting point | 283-286 °C(lit.) |
|---|---|
| Boiling point | 724.3±55.0 °C(Predicted) |
| alpha | 240 º (c=0.3, toluene) |
| refractive index | 235 ° (C=0.3, Toluene) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Benzene (Slightly), Chloroform (Slightly) |
| form | Powder |
| color | White to cream-white |
| optical activity | [α]20/D +222°, c = 0.5% in benzene |
| Water Solubility | insoluble |
| Sensitive | Air Sensitive |
| Merck | 14,1223 |
| BRN | 4914063 |
| CAS DataBase Reference | 76189-55-4(CAS DataBase Reference) |
| FDA UNII | 4F1X2F8NA3 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P280a-P304+P340-P305+P351+P338-P405-P501a | |||||||||
| Hazard Codes | Xi,Xn | |||||||||
| Risk Statements | 36/37/38-20/21/22 | |||||||||
| Safety Statements | 22-24/25-37/39-26-36 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 8-10-23 | |||||||||
| TSCA | No | |||||||||
| HS Code | 29319090 | |||||||||
| NFPA 704 |
|
(R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl price More Price(54)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 693065 | (R)-BINAP | 76189-55-4 | 100MG | $18.78 | 2024-03-01 | Buy |
| Sigma-Aldrich | 693065 | (R)-BINAP | 76189-55-4 | 500MG | $79.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 295817 | (R)-(+)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthalene 97% | 76189-55-4 | 1g | $54 | 2024-03-01 | Buy |
| Sigma-Aldrich | 295817 | (R)-(+)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthalene 97% | 76189-55-4 | 5g | $143 | 2024-03-01 | Buy |
| TCI Chemical | B1406 | (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl | 76189-55-4 | 1g | $88 | 2024-03-01 | Buy |
(R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl Chemical Properties,Uses,Production
Reaction
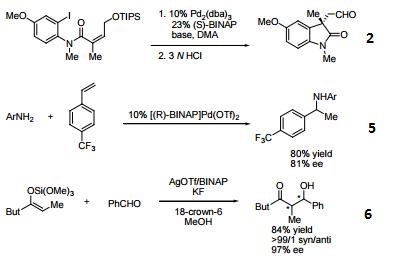
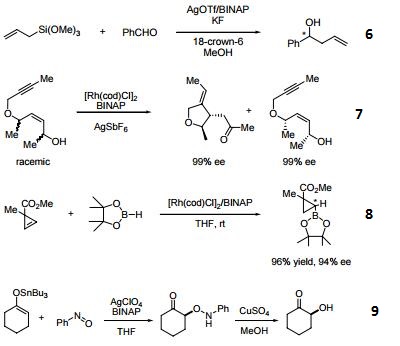
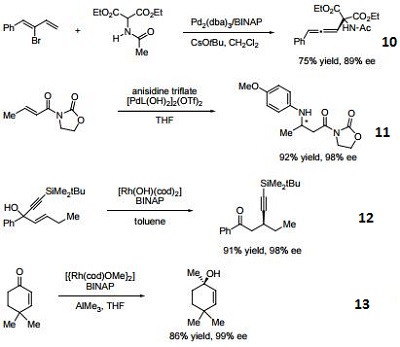
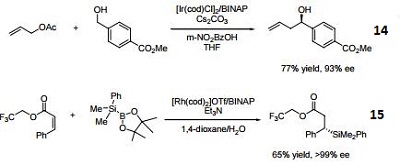
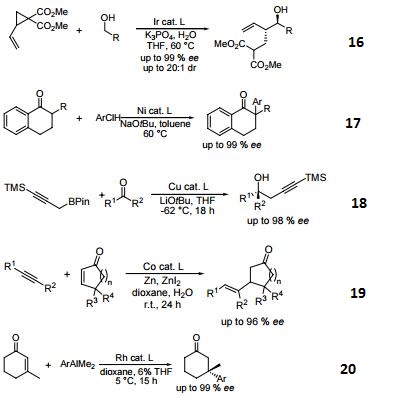
- (R)-BINAP or (R)-Tol-BINAP can be combined with dichloro(1,5-cyclooctadiene)ruthenium to form precursors to NOYORI CATALYST SYSTEMS. These systems exhibit very high catalytic activity and enantioselectivity in the hydrogenation of a wide range of substrates. NOYORI CATALYST SYSTEMS have been shown to effect highly enantioselective hydrogenation of functionalized ketones where the substituents are dialkylamino, hydroxy, siloxy, carbonyl, ester, amide or thioester.
- Useful ligand in asymmetric Heck processes.
- Ligand employed in palladium-catalyzed asymmetric arylation of ketones.
- Ligand employed in rhodium-catalyzed 1,4-additions to enones.
- Ligand employed in palladium-catalyzed hydroamination of styrene derivatives.
- Ligand employed in silver-catalyzed asymmetric Sakuri-Hosomi allylation and Mukaiyama aldol reaction.
- Ligand employed in rhodium-catalyzed kinetic resolution of enynes.
- Ligand employed in asymmetric rhodium-catalyzed hydroboration of cyclopropenes.
- Ligand employed in silver-catalyzed a-hydroxylation of stannyl enol ethers.
- Ligand employed in palladium-catalyzed synthesis of chiral allenes.
- Ligand for palladium-catalyzed enantioselective hetero Michael addition to form b-amino acid derivatives.
- Ligand employed in rhodium-catalyzed asymmetric rearrangement of alkynyl alkenyl carbinols.
- Ligand employed in rhodium-catalyzed 1,2-addition of aluminium organyl compounds to cyclic enones.
- Ligand employed in iridium-catalyzed transfer hydrogenative allylation of benzylic alcohols.
- Ligand employed in rhodium-catalyzed asymmetric C-Si bond formation by conjugate silyl transfer using a Si-B linkage.
- Ligand employed in the iridium-catalyzed asymmetric cyclopropane-mediated carbonyl allylation of primary alcohols.
- Ligand employed in the nickel-catalyzed asymmetric α-arylation of tetralones.
- Ligand employed in the copper-catalyzed asymmetric propargylation of ketones.
- Ligand employed in the cobalt-catalyzed asymmetric reductive coupling of alkynes with alkenes.
- Ligand employed in the rhodium-catalyzed asymmetric 1,4-addition of arylalanes on trisubstituted enones.
- Ruthenium-catalyzed asymmetric hydrocyanation of imines.
- Palladium-catalyzed asymmetric intermolecular cyclization.
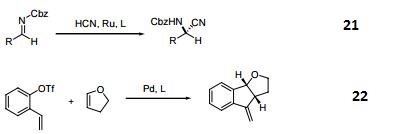
Chemical Properties
white to light yellow crystal powde
Uses
Useful ligand for transition metal catalyzed asymmetric reactions, including hydrogenation and disilylation. 2,2′-Bis(diphenylphosphino)-1,1′-binaphthyl and its rhodium and ruthenium derivatives are highly selective homogeneous catalysts used for the reduction of aryl ketones, β-keto esters, and α-amino ketones. They have also been used for asymmetric hydrogenation and hydroformylation of olefins, asymmetric Heck reactions, and asymmetric isomerizations of allyls.
Complex with Ag(I) used to catalyze an asymmetric aldol reaction between alkenyl trichloroacetates and aldehydes. Also used with Ag(I) to catalyze an enantioselective hetero-Diels-Alder reaction of azo compounds.
Uses
Chiral ligand for metal mediated asymmetric catalysis.
General Description
(R)-(+)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthalene is an axially dissymmetric bis(triaryl)phosphine ligand for asymmetric reactions.
(R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| XINXIANG RUNYU MATERIAL CO., LTD. | +86-13592593621; +8613592593621 | sales@runvmat.com | China | 402 | 58 |
| Zhengzhou HQ Material CO.,LTD. | +86-371-55919009 +8615225108189 | sale1@hqmat.com | China | 633 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 | sales@myuchem.com | China | 6710 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
Related articles
- (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl: properties and applications in organic synthesis
- (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl is a crucial and versatile chiral ligand in asymmetric catalysis, enabling....
- Nov 23,2023
- The applications and synthesize of 2-2-bis-diphenylphosphino-1-1-binaphthyl
- BINAP (2,2'-bis(diphenylphosphino)-1,1'-binaphthyl) is an organophosphorus compound. This chiral ligand is widely used in asym....
- Aug 19,2019
View Lastest Price from (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-02-23 | (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl
76189-55-4
|
US $230.00-1005.00 / g | 1g | 0.98 | 25kg | Shanghai UCHEM Inc. | |
 |
2023-07-28 | (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl
76189-55-4
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-07-27 | (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl
76189-55-4
|
US $0.00-0.00 / KG | 1KG | 0.99 | 500kg | XINXIANG RUNYU MATERIAL CO., LTD. |
-

- (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl
76189-55-4
- US $230.00-1005.00 / g
- 0.98
- Shanghai UCHEM Inc.
-

- (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl
76189-55-4
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl
76189-55-4
- US $0.00-0.00 / KG
- 0.99
- XINXIANG RUNYU MATERIAL CO., LTD.
(R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl Spectrum
76189-55-4((R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl)Related Search:
1of4