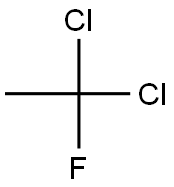
Dichlorofluoroethane
- CAS No.
- 1717-00-6
- Chemical Name:
- Dichlorofluoroethane
- Synonyms
- 1,1-DICHLORO-1-FLUOROETHANE;r141b;F141b;HCFC 141B;Freon 141b;R 141b;FC-141B;cfc141b;DF-141b;HFC141B
- CBNumber:
- CB2217640
- Molecular Formula:
- C2H3Cl2F
- Molecular Weight:
- 116.95
- MDL Number:
- MFCD00042461
- MOL File:
- 1717-00-6.mol
| Melting point | -104°C |
|---|---|
| Boiling point | 32°C |
| Density | 1.25 |
| refractive index | 1.3600 |
| Stability | Stable. |
| CAS DataBase Reference | 1717-00-6(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | O1A3ASY5PW |
| NIST Chemistry Reference | 1,1-Dichloro-1-fluoroethane(1717-00-6) |
| EPA Substance Registry System | HCFC-141b (1717-00-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H412-H420 | |||||||||
| Precautionary statements | P273-P501-P502 | |||||||||
| Hazard Codes | Xi,N | |||||||||
| Risk Statements | 59-52/53 | |||||||||
| Safety Statements | 23-36/37/39-61-59 | |||||||||
| RIDADR | 3082 | |||||||||
| Hazard Note | Irritant | |||||||||
| IDLA | 1,700 ppm (8,245 mg/m3) | |||||||||
| NFPA 704 |
|
Dichlorofluoroethane Chemical Properties,Uses,Production
Chemical Properties
colourless liquid
Uses
HCFC 141b has been developed as a substitute for the fully halogenated chlorofluorocarbons, mainly for use as a blowing agent for polyurethane and polyisocyanate insulating foams and as a solvent in electronic and other precision cleaning applications. It is viewed as an interim compound, and production will be phased out beginning in 2003.
General Description
Colorless liquid at ambient conditions.
Reactivity Profile
Dichlorofluoroethane may be incompatible with strong oxidizing and reducing agents. May be incompatible with some amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. An explosion occurred when an emptied drum of Dichlorofluoroethane was cut into by a grinder; may be more flammable than once thought.
Carcinogenicity
A 2 year inhalation study was conducted during which four groups of 80 male and 80 female rats were exposed to levels of 0, 1500, 5000, and 15,000–20,000 ppm, 6 h/day, 5 days/week. The high exposure level was increased from 15,000 to 20,000 ppm after 4 months since no signs of toxicity were seen at 15,000 ppm. At the end of 2 years, an increased incidence of benign Leydig cell tumors and Leydig cell hyperplasia was seen in the male rats 5000 and 20,000 ppm, but not at 1500 ppm. These tumors are common in the aging rat and did not follow an exposure related pattern. They are not considered to be a risk factor for humans. No other treatment related effects were seen.
Dichlorofluoroethane Preparation Products And Raw materials
Raw materials
Preparation Products
Related articles
- Preparation of Dichlorofluoroethane
- Dichlorofluoroethane (HCFC-141b, 1,1-dichloro-1-fluoroethane) is an organic solvent and an important amethylating agent.
- Nov 5,2019