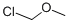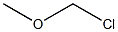Chloromethyl methyl ether
- CAS No.
- 107-30-2
- Chemical Name:
- Chloromethyl methyl ether
- Synonyms
- CH3OCH2Cl;chloromethyl;METHOXYMETHYL CHLORIDE;chloromethoxymethane;CMME;METHYL CHLOROMETHYL ETHER;MOM chloride;Chloromethyl methyl;Methoxychloromethane;chlormethyl methyl ether chlorodimethyl ether
- CBNumber:
- CB2303754
- Molecular Formula:
- C2H5ClO
- Molecular Weight:
- 80.51
- MDL Number:
- MFCD00000885
- MOL File:
- 107-30-2.mol
| Melting point | -103°C |
|---|---|
| Boiling point | 55-57 °C(lit.) |
| Density | 1.06 g/mL at 25 °C(lit.) |
| vapor pressure | 3.55 psi ( 20 °C) |
| refractive index |
n |
| Flash point | 60 °F |
| storage temp. | 0-6°C |
| Water Solubility | decomposes |
| Merck | 13,2165 |
| CAS DataBase Reference | 107-30-2(CAS DataBase Reference) |
| EWG's Food Scores | 7 |
| NIST Chemistry Reference | Methane, chloromethoxy-(107-30-2) |
SAFETY
Risk and Safety Statements
| Hazard Codes | F,T | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Risk Statements | 45-11-20/21/22 | |||||||||
| Safety Statements | 53-45 | |||||||||
| RIDADR | UN 1239 6.1/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | KN6650000 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | I | |||||||||
| NFPA 704 |
|
Chloromethyl methyl ether Chemical Properties,Uses,Production
Chemical Properties
Clear Colourless Oil
Uses
A chloroalkyl ether that is an efficient alkylating agent. Used as an alcohol protecting group in the preparation of various organic compounds. A known human carcinogen.
Definition
ChEBI: Chloromethyl methyl ether is an ether.
General Description
A clear colorless liquid. Flash point -4°F. Irritates the eyes and respiratory system. Very toxic by inhalation and may be toxic by ingestion or skin absorption. Vapors are heavier than air.
Air & Water Reactions
Highly flammable. Denser than water and is decomposed by water to yield hydrochloric acid, a corrosive material. With water the ether reacts to evolve formaldehyde and hydrogen chloride. The reaction is slow at ambient conditions.
Reactivity Profile
Chloromethyl methyl ether Is a halogenated ether. Ethers tend to form unstable peroxides when exposed to oxygen. Ethyl, isobutyl, ethyl tert-butyl, and ethyl tert-pentyl ether are particularly hazardous in this respect. Ether peroxides can sometimes be observed as clear crystals deposited on containers or along the surface of the liquid. Ethers can act as bases. They form salts with strong acids and addition complexes with Lewis acids. The complex between diethyl ether and boron trifluoride is an example. Ethers may react violently with strong oxidizing agents. In other reactions, which typically involve the breaking of the carbon-oxygen bond, ethers are relatively inert.
Health Hazard
The principal effect is irritation. The liquid causes severe irritation of eyes and skin; and vapor exposure of 100 ppm is severely irritating to eyes and nose. This level is dangerous to life in 4 hours. Pulmonary edema or pneumonia may cause death. There was increased death rate from respiratory cancer among exposed victims and it is a regulated carcinogen.
Fire Hazard
Flammable/combustible material; may be ignited by heat, sparks, or flames. Vapors may travel to a source of ignition and flash back. Container may explode in heat of fire. In addition to the risk of explosion, when air mixtures of ether vapors are heated or exposed to flame or sparks, they tend to form peroxides. Ethers containing peroxides can detonate when heated. Unburned material may form powerful tear gas. When wet, also forms irritating formaldehyde gas. Evolves formaldehyde and hydrogen chloride. When heated to decomposition, Chloromethyl methyl ether emits toxic fumes of chlorides. Avoid decomposing heat Hazardous polymerization may not occur.
Chloromethyl methyl ether Preparation Products And Raw materials
Raw materials
Preparation Products
1of2