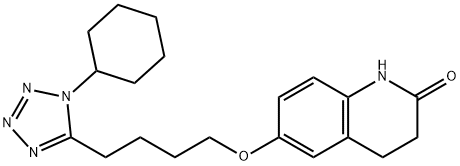
Cilostazol
- CAS No.
- 73963-72-1
- Chemical Name:
- Cilostazol
- Synonyms
- RETAL;OPC 21;PLETAL;PLETAAL;Cilostal;OPC 13013;Cilostazo;CILASTAZOL;CILOSTAZOL;CILOSTAZOLE
- CBNumber:
- CB2362876
- Molecular Formula:
- C20H27N5O2
- Molecular Weight:
- 369.46
- MDL Number:
- MFCD00866780
- MOL File:
- 73963-72-1.mol
- MSDS File:
- SDS
| Melting point | 159-160°C |
|---|---|
| Boiling point | 499.57°C (rough estimate) |
| Density | 1.1832 (rough estimate) |
| refractive index | 1.7600 (estimate) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | DMSO: 18 mg/mL, soluble |
| form | solid |
| pka | 14.22±0.20(Predicted) |
| color | off-white |
| λmax | 257nm(MeOH)(lit.) |
| Merck | 14,2277 |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 2 months |
| InChIKey | RRGUKTPIGVIEKM-UHFFFAOYSA-N |
| FDA UNII | N7Z035406B |
| ATC code | B01AC23 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H361d |
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501 |
| Hazard Codes | Xi |
| WGK Germany | 2 |
| RTECS | VC8277500 |
| HS Code | 29339900 |
| Toxicity | LD50 in mice, rats (mg/kg): >2000, >2000 i.p.; >5000, >5000 orally (Nomura) |
Cilostazol price More Price(44)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | C0737 | Cilostazol ≥98% (HPLC), powder | 73963-72-1 | 10mg | $212 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1134153 | Cilostazol United States Pharmacopeia (USP) Reference Standard | 73963-72-1 | 200mg | $1090 | 2024-03-01 | Buy |
| TCI Chemical | C2587 | Cilostazol >98.0%(HPLC) | 73963-72-1 | 1g | $105 | 2024-03-01 | Buy |
| TCI Chemical | C2587 | Cilostazol >98.0%(HPLC) | 73963-72-1 | 5g | $355 | 2024-03-01 | Buy |
| Alfa Aesar | J62301 | Cilostazol, 98% | 73963-72-1 | 10mg | $120.65 | 2024-03-01 | Buy |
Cilostazol Chemical Properties,Uses,Production
Pharmacological effects
Cilostazol is commonly used clinically anti-platelet and anti-clotting drugs. It belongs to a phosphodiesterase inhibitor and can inhibit the activity of phosphodiesterase of platelet and smooth muscle cells, leading to increase of the cAMP concentration in platelets and vascular smooth muscle. It can significantly inhibit the platelet aggregation induced by various kinds of aggregation inducers, and can cause the dissociation of the aggregates. Its major metabolite, epoxide has a three to four fold activity of the prototype drug. It has significant antithrombotic effect on the brain circulation and peripheral circulatory disturbance caused by collagen, ADP, arachidonic acid and sodium laurate. Artery injection of this product can increase the blood flow rate with the strongest effect on the peripheral blood vessels but the weakest effect on the cerebral blood vessels.
This product is rapidly absorbed in the intestine after oral administration with the Tmax being 3h, PPB being about 95%. Consecutive administration of four days by twice per day caused no increase in the plasma concentration accumulation. It has good tissue distribution with especially high level in the stomach, liver and kidney. At 72h after administration, 42.75% of the administered drug is excreted via urine and 61.7% is subject to fecal excretion. After 48 hour of administration, bile excretion rate is 31.7%. T1/2α is 2.2h and T1/2β is 18h.
Clinical application
It can be used to alleviate the ischemic symptom such as ulcers, limb pain, cold sensation and intermittent claudication caused by chronic arterial occlusive disease. It can also be applied to the adjuvant treatment of atherosclerosis, arteritis, thromboangiitis obliterans, diabetes-induced ischemia of extremity and takayasu arteritis. Cilostazol also be used as a complementary therapy after surgical treatment to help alleviate the symptoms, improve the circulation and inhibiting thrombosis in transplanted blood vessel.
Side effects
There may be occasionally rash, hives, itching, palpitations, pulse frequency, low blood pressure, fever, dizziness, dizziness, vertigo, insomnia or drowsiness, swelling, pain, fatigue, weakness, stomach discomfort, nausea, vomiting, loss of appetite, diarrhea, upper abdominal pain, abdominal fullness, increased level of GOT (serum alanine aminotransferase), ALP (serum alkaline phosphatase), LDH (serum lactate dehydrogenase), BUN (blood urea nitrogen), creatinine and uric acid, gastrointestinal bleeding, epistaxis, subcutaneous bleeding, retinal hemorrhage, hematuria, bleeding tendencies and thrombocytopenia.
The above information is edited by the chemicalbook of Dai Xiongfeng.
Precautions
Patients of hemophilia, capillary fragility syndrome, upper gastrointestinal bleeding and urinary tract bleeding as well as vitreous hemorrhage should be disabled. Women of pregnancy or possible pregnancy should be disabled.
This product is should be taken cautious when being applied to patients in the use of anticoagulants (warfarin) or antiplatelet drugs (aspirin, Ticlid) patients. When using cilostazol, the patients should be subject to blood coagulation performance test.
Women in menstrual period, patients of bleeding tendency or with severe hepatic and renal dysfunction as well as elderly should administer with caution; breast-feeding women should avoid breast-feeding.
Chemical Properties
It is colorless, needle-like crystals obtained from methanol with the m.p. being 159.4~160.3 ℃ and the UV absorption maximum (methanol) being 257nm (ε15200). It is easily soluble in acetic acid, chloroform, N-methyl-2-pyrrolidone or dimethyl sulfoxide and almost insoluble in ether, water, 0.1mol/L hydrochloric acid or 0.1mol/L sodium hydroxide.
Uses
It can significantly inhibit the platelet aggregation caused by various inducers and aggregates and can dissociate the aggregates without causing secondary aggregation. It has significant antithrombotic effect on the brain circulation and peripheral circulatory disturbance caused by collagen, ADP, arachidonic acid and sodium laurate. It can also be used for treating ischemic diseases such as chronic arterial occlusive ulcer, pain and coldness.
Production method
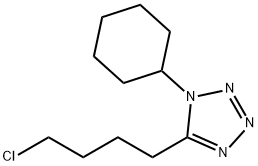
Take 5-chloro-N-cyclohexyl pentanamide as the raw material. Under ice-cooling, to 15 mL of benzene solution containing 1.75g 5-chloro-N-cyclohexyl-pentyl amide solution, slowly add 1.9 g of phosphorus pentachloride and stir at room temperature for 1h. At room temperature and stirring, add 1.4mol/L HN3 to 1 mL of the benzene solution. After stirring overnight, continue the reflux for 2h. The solvent was distilled off under reduced pressure with the residue being poured into ice water and subject to chloroform extraction. The extract was successively washed with water, dilute sodium bicarbonate solution and water, further dried by anhydrous sodium sulfate. After the chloroform was distilled off, the residue was subject to isopropanol-water recrystallization to obtain 1.7 g of 5-(4-chlorobutyl)-l-cyclohexyl-tetrazole, being as colorless needle-like crystals with the yield being 87% and the m.p. being 48-49 ℃.
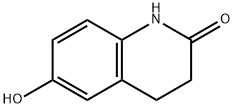
Dissolve 3.2 g 6-hydroxy-3, 4-dihydrogen-2(1H)-quinolinone and 1.4 g of potassium hydroxide in 20 mL of isopropanol. Under reflux, add drop wise of the isopropanol solution containing 5.7 g of the tetrazole obtained above. Continue stirring and reflux for 4h. Evaporate to dryness with the residue being extracted with chloroform. The extract was washed with l mol/L sodium hydroxide solution, dilute hydrochloric acid and water, dried by anhydrous sodium sulfate. Chloroform was distilled off with the residual liquid being subject to chromatograph on silica gel. Use chloroform-methanol (30: 1) for elution. Then apply methanol-water recrystallization to obtain 6.0 g of colorless needles cilostazol with the yield being 74% and the melting point being 158 ~ 159 ℃.
Description
Cilostazol is a platelet aggregation inhibitor with cerebral vasodilating activity, indicated for use in stroke and myocardial infarction. In patients with cerebral thrombosis, transient ischemia and cerebral arteriosclerosis, cilostazol significantly inhibits ADP-, collagenand epinephrine-induced platelet aggregation. Side effects include headache and tachycardia.
Chemical Properties
Colourless Needles
Originator
Otsuka (Japan)
Uses
An inhibitor of phosphodiesterase III
Uses
antibacterial; LD50(iv) 280mg/kg(mouse)
Uses
A potent phosphodiesterase III A (PDE3A) inhibitor (IC50=0.2uM) and inhibitor of adenosine uptake. Has antimitogeni, antithrombotic, vasodilatory and cardiotonic properties in vivo. Also affects lipid levels in vivo
Definition
ChEBI: A lactam that is 3,4-dihydroquinolin-2(1H)-one in which the hydrogen at position 6 is substiuted by a 4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy group.
brand name
Pletal (Otsuka);Reta.
General Description
Cilostazol is a potent cyclic nucleotide phosphodiesterase inhibitor. It is mainly used as antiplatelet agent.
Biological Activity
Potent phosphodiesterase III A (PDE3A) inhibitor (IC 50 = 0.2 μ M) and inhibitor of adenosine uptake. Has antimitogenic, antithrombotic, vasodilatory and cardiotonic properties in vivo . Also affects lipid levels in vivo .
Mechanism of action
Cilostazol exhibits greater selectivity than dipyridamole as an inhibitor of PDE3A. The drug does not affect the other PDEs (PDEs 1, 2, or 4). Cilostazol reversibly inhibit platelet aggregation induced by a number of stimuli, such as thrombin, ADP, collagen, or stress from exercise. Additionally, cilostazol inhibits adenosine uptake, leading to increased activity of adenosine at A1 and A2 receptors.
Clinical Use
Cilostazol, a quinolinone derivative, is a potent orally active antiplatelet drug approved for the treatment of intermittent claudication (a peripheral artery disease resulting from blockage of blood vessels in the limbs).
Drug interactions
Potentially hazardous interactions with other drugs
Anagrelide: avoid concomitant use. Antibacterials: concentration increased by
clarithromycin and erythromycin - consider
reducing cilostazol dose.
Antifungals: concentration possibly increased by
ketoconazole and itraconazole - consider reducing
cilostazol dose.
Antivirals: concentration possibly increased by
boceprevir, ritonavir and telaprevir - reduce
cilostazol dose to 50 mg twice daily.
Calcium-channel blockers: concentration increased
by diltiazem - consider reducing cilostazol dose.
Ulcer-healing drugs: concentration increased by
omeprazole - consider reducing cilostazol dose.
Metabolism
Cilostazol is rapidly absorbed after oral administration, particularly with a high-fat meal, which greatly increases its bioavailability to approximately 90%. It is extensively metabolized in the liver by various cytochromes. The most important cytochromes appear to be CYP3A4 and, to lesser extent, by CYP2C19, with an elimination half-life of approximately 11 to 13 hours. Among the various metabolites produced (11 metabolites are known), the two major metabolites are 3,4-dehydrocilostazol and 4′-trans-hydroxycliostazol. These two metabolites are pharmacologically active. Studies indicate that the concomitant administration of cilostazol with CYP3A inhibitors can greatly increase cilostazol blood concentrations, and a dose reduction may be required. Similar results are seen when CYP2C19 is inhibited, leading to decreased formation of 4-trans-hydroxycliostazol and significant increases in cilostazol and 3,4-dehydrocilostazol.
storage
Store at RT
References
1) Schror (2002) The pharmacology of cilostazol; Diabetes Obes. Metab., 4 S14
Cilostazol Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Shanghai Affida new material science and technology center | +undefined15081010295 | 2691956269@qq.com | China | 359 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
View Lastest Price from Cilostazol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-03-16 | Cilostazol
73963-72-1
|
US $0.00 / KG | 100g | 98%+ | 100kg | WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD | |
 |
2024-03-13 | Cilostazol
73963-72-1
|
US $0.00-0.00 / kg | 1kg | 99 | 50000KG/month | Shanghai Affida new material science and technology center | |
 |
2023-08-02 | Cilostazol
73963-72-1
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd |
-

- Cilostazol
73963-72-1
- US $0.00 / KG
- 98%+
- WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD
-

- Cilostazol
73963-72-1
- US $0.00-0.00 / kg
- 99
- Shanghai Affida new material science and technology center
-

- Cilostazol
73963-72-1
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
73963-72-1(Cilostazol)Related Search:
1of4





