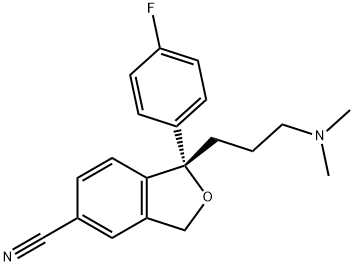
Escitalopram
- CAS No.
- 128196-01-0
- Chemical Name:
- Escitalopram
- Synonyms
- Seroplex;Escitalopram;Escitalopram CRS;EscitalopraM Oxalate INN;Escitalopram Impurity 31;(S)-(+)-CITALOPRAM OXALATE;(+)-(S)-Citalopram Oxalate;(+)-(S)-Citalopram-d4 Oxalate;Escitalopram oxalate impurity III;Escitalopram(Escitalopram Oxalate)
- CBNumber:
- CB2712918
- Molecular Formula:
- C20H21FN2O
- Molecular Weight:
- 324.39
- MDL Number:
- MFCD09817397
- MOL File:
- 128196-01-0.mol
- MSDS File:
- SDS
| alpha | D +12.33° (c = 1 in methanol) |
|---|---|
| Boiling point | 428.3±45.0 °C(Predicted) |
| Density | 1.18±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO |
| form | Powder |
| pka | 9.57±0.28(Predicted) |
| CAS DataBase Reference | 128196-01-0(CAS DataBase Reference) |
| FDA UNII | 4O4S742ANY |
| NCI Dictionary of Cancer Terms | escitalopram |
| ATC code | N06AB10 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H317-H302 | |||||||||
| Precautionary statements | P261-P272-P280-P302+P352-P333+P313-P321-P363-P501-P264-P270-P301+P312-P330-P501 | |||||||||
| NFPA 704 |
|
Escitalopram price More Price(17)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 22405 | Escitalopram ≥98% | 128196-01-0 | 5mg | $45 | 2024-03-01 | Buy |
| Cayman Chemical | 22405 | Escitalopram ≥98% | 128196-01-0 | 10mg | $81 | 2024-03-01 | Buy |
| Cayman Chemical | 22405 | Escitalopram ≥98% | 128196-01-0 | 25mg | $187 | 2024-03-01 | Buy |
| Cayman Chemical | 22405 | Escitalopram ≥98% | 128196-01-0 | 50mg | $330 | 2024-03-01 | Buy |
| Usbiological | C5670-15E | Escitalopram | 128196-01-0 | 500ul | $523 | 2021-12-16 | Buy |
Escitalopram Chemical Properties,Uses,Production
Uses
(S)-Citalopram is an inhibitor of serotonin (5-HT) uptake. Antidepressant.
Uses
antidepressive;selective serotonin reuptake inhibitor
Definition
ChEBI: A 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-2-benzofuran-5-carbonitrile that has S-configuration at the chiral centre. It is the active enantiomer of citalopram.
Biological Activity
escitalopram (lexapro,cipralex)is a selective inihibitor of serotonin(5-ht) reuptake (ssri) with a ki value of 6.6nm for [3h]-5-ht uptake [1].escitalopram is the s-(+)-enantiomer of citalopram and has the inhibition of [3h]-5-ht uptake and [125i]-rti-55 binding in cos-1 cells expressing the human serotonin transporter (5-htt) with ki values of 6.6±1.4nm and 3.9±2.2nm, respectively. in addition, escitalopram has been reported to inhibit the accumulation of 3h-labelled monoamines into rat brain synaptosomes in vitro with ic50 values of 2.1±0.75nm, 2500±202nm and 40000±15500nm for [3h]-5-ht, [3h]-i-na and [3h]-da, respectively. apart from these, escitalopram has shown the in vitro affinity for rat histamine h1 receptors and the rat sigma σ1 site with ki values of 1500±780nm and 100±71nm, respectively [1].
Mechanism of action
Escitalopram is the S-enantiomer of citalopram that binds with high affinity and selectivity to the human SERT equivalent to (±)-citalopram. It has been reported that nearly all the activity resides in the S-enantiomer and that R-citalopram actually counteracts the action of the S-enantiomer. Studies show that escitalopram exhibits twice the activity of citalopram and is at least 27 times more potent than the R-enantiomer. The R-enantiomer inhibits the S-enantiomer at the transporter. Escitalopram's mechanism of action is common to the SSRIs.
Pharmacokinetics
The pharmacokinetics for escitalopram does not exhibit stereoisomer selectivity and, therefore, is similar to that for citalopram. Likewise, it exhibits linear pharmacokinetics so that plasma levels increase proportionately and predictably with increased doses, and its half-life of 27 to 32 hours is consistent with once-daily dosing. It also has been found that R-citalopram is cleared more slowly than the S-enantiomer. Therefore, when the drug is used as a racemic mixture (citalopram), the inactive isomer predominates at steady state. This is an added incentive for use of the enantiomerically pure escitalopram. Escitalopram has negligible effects on CYP isoforms, suggesting a low potential for drug–drug interactions. Escitalopram is indicated for patients with major depressive disorder, generalized anxiety disorder, panic disorder, and social anxiety disorder.
Clinical Use
SSRI antidepressant:
Depressive illness
Panic and social anxiety disorder
Drug interactions
Potentially hazardous interactions with other drugsAnalgesics: increased risk of bleeding with aspirin and NSAIDs; risk of CNS toxicity increased with tramadol.Anti-arrhythmics: increased risk of ventricular arrhythmias with amiodarone, disopyramide and dronedarone - avoid.Antibacterials: increased risk of ventricular arrhythmias with IV erythromycin, moxifloxacin, pentamidine and telithromycin.Anticoagulants: effect of coumarins possibly enhanced; possibly increased risk of bleeding with dabigatran.Antidepressants: avoid concomitant use with MAOI, increased risk of toxicity; increased risk of CNS toxicity with moclobemide - avoid concomitant use; avoid concomitant use with St John’s wort; possibly enhanced serotonergic effects with dapoxetine and duloxetine; can increase concentration of tricyclics; increased agitation and nausea with tryptophan; increased risk of CNS toxicity with rasagiline; possible increased risk of convulsions with vortioxetineAntiepileptics: convulsive threshold lowered.Antihistamines: increased risk of ventricular arrhythmias with mizolastine - avoid.Antimalarials: avoid concomitant use with artemether/lumefantrine and piperaquine with artenimol; possible increased risk of ventricular arrhythmias with chloroquine and quinine.Antipsychotics: possibly increased risk of ventricular arrhythmias with haloperidol, phenothiazines and pimozide - avoid.Antivirals: concentration possibly increased by ritonavir.Beta-blockers: increased risk of ventricular arrhythmias with sotalol - avoid.Dopaminergics: avoid with selegiline; increased risk of CNS toxicity with rasagiline.5HT1 agonist: increased risk of CNS toxicity with sumatriptan; possibly increased risk of serotonergic effects with naratriptan.Linezolid: use with care, possibly increased risk of side effects.Lithium: increased risk of CNS effectsMethylthioninium: risk of CNS toxicity - avoid if possible.
Metabolism
Escitalopram is metabolised in the liver to the demethylated and didemethylated metabolites. Both of these are pharmacologically active. Alternatively, the nitrogen may be oxidised to form the N-oxide metabolite. Both parent substance and metabolites are partly excreted as glucuronides. After multiple dosing the mean concentrations of the demethyl and didemethyl metabolites are usually 28-31% and <5%, respectively, of the escitalopram concentration. Biotransformation of escitalopram to the demethylated metabolite is mediated primarily by CYP2C19. Some contribution by the enzymes CYP3A4 and CYP2D6 is possible. The major metabolites have a significantly longer half-life than the parent drug.Escitalopram and major metabolites are assumed to be eliminated by both hepatic and renal routes, with the major part of the dose excreted as metabolites in the urine.
References
[1] sánchez c1, bergqvist pb, brennum lt, gupta s, hogg s, larsen a, wiborg o. escitalopram, the s-(+)-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with potent effects in animal models predictive of antidepressant and anxiolytic activities. psychopharmacology (berl). 2003 jun;167(4):353-62. epub 2003 apr 26.
Escitalopram Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10326 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6313 | 58 |
| Xi'an MC Biotech, Co., Ltd. | 029-89275612 +8618991951683 | mcbio_sales@163.com | China | 2255 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
View Lastest Price from Escitalopram manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-03-27 | Escitalopram
128196-01-0
|
US $25.00 / KG | 1KG | 0.99 | 50000kg/month | XINGTAI XINGJIU NEW MATERIAL TECHNOLOGY CO., LTD | |
 |
2020-01-10 | Escitalopram
128196-01-0
|
US $1.00 / KG | 1g | 98% | 200kgs | Career Henan Chemical Co |
-

- Escitalopram
128196-01-0
- US $25.00 / KG
- 0.99
- XINGTAI XINGJIU NEW MATERIAL TECHNOLOGY CO., LTD
-

- Escitalopram
128196-01-0
- US $1.00 / KG
- 98%
- Career Henan Chemical Co