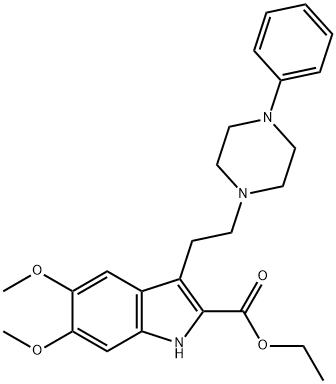
Alpertine
- CAS No.
- 27076-46-6
- Chemical Name:
- Alpertine
- Synonyms
- Alpertine;WIN-31665;5,6-Dimethoxy-3-[2-(4-phenyl-1-piperazinyl)ethyl]-1H-indole-2-carboxylic acid ethyl ester;1H-Indole-2-carboxylic acid, 5,6-dimethoxy-3-[2-(4-phenyl-1-piperazinyl)ethyl]-, ethyl ester
- CBNumber:
- CB31177979
- Molecular Formula:
- C25H31N3O4
- Molecular Weight:
- 437.537
- MDL Number:
- MFCD00866655
- MOL File:
- 27076-46-6.mol
| Melting point | 142.5-144 °C |
|---|---|
| Boiling point | 616.5±55.0 °C(Predicted) |
| Density | 1.195±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| pka | 15.23±0.30(Predicted) |
| FDA UNII | KYT38QTB4K |
Alpertine Chemical Properties,Uses,Production
Originator
Alpertine ,ZYF Pharm Chemical
Uses
Antipsychotic.
Definition
ChEBI: Alpertine is a member of piperazines.
Manufacturing Process
1-[2-(2-Carbethoxy-5,6-dimethoxy-3-indolyl)ethyl]-4-phenylpiperazine:
To a suspension of 23 g (1.0 mole) of sodium pellets in 800 ml of absolute
ether was added 80 ml of a mixture of 86 g (1.0 mole) of γ-butyrolactone and
146 g (1.0 mole) of ethyl oxalate. The reaction mixture began to boil gently
and was allowed to reflux spontaneously for two hours, after which time the
remainder of the γ-butyrolactone and ethyl oxalate mixture was added cautiously. When addition was complete, the mixture was refluxed for one
hour, allowed to stand overnight, and the ether removed in vacuo. The residue
was mixed with ice, acidified with cold, dilute sulfuric acid, extracted with
ether, and the ether extracts dried over sodium sulfate and taken to dryness.
Distillation of the residue in vacuo at 0.05 mm afforded 98 g of α-ethoxalyl-γ-
butyrolactone, collected between 110-126°C.
Forty grams (0.215 mole) of the latter were heated under reflux in 100 ml of
2 N sulfuric acid until the evolution of carbon dioxide ceased, giving a solution
of α-keto-δ-valerolactone.
3,4-Dimethoxyphenylhydrazine hydrochloride (44 g, 0.22 mole) was dissolved
in 300 ml of water, treated with a solution of 12.3 g (0.22 mole) of potassium
hydroxide in 50 ml of water, and cooled. To this mixture was added the above
described solution of α-keto-δ-valerolactone, and the pH of the mixture was
adjusted to about 2 with 10% sodium hydroxide. The mixture was warmed on
a hot plate for five minutes, allowed to cool, extracted with chloroform, and
the extracts dried over magnesium sulfate and concentrated to dryness giving
66 g of crude hydrazone.
The latter was dissolved in 100 ml of absolute ethanol, the mixture acidified
with 400 ml of saturated ethanolic hydrogen chloride, and a stream of
hydrogen chloride gas was passed through the mixture causing the
temperature to rise to 80°C. The solid which separated from the reaction
mixture was collected after standing overnight, and washed with cold absolute
ethanol to give 38 g of crude 2-carboxy-5,6 -dimethoxy-3-(2-
hydroxyethyl)indole.
The latter was suspended in 300 ml of absolute ethanol and the solution
saturated with anhydrous hydrogen chloride for one hour. The mixture was
allowed to stand for two hours, and the solid which separated was collected
and dried to give 24 g of 2-carbethoxy-5,6-dimethoxy-3-(2-chloroethyl)indole,
M.P. 179-181°C.
The latter was added to 15 ml of 1-phenylpiperazine and the mixture heated
at 140-160°C for one hour and twenty minutes. The cooled mixture was
triturated with 100 ml of ether, filtered, and the ether filtrate concentrated to
dryness. The residue was mixed with water and acetic acid, the pH adjusted
to about 5.0, and the insoluble material was collected by filtration giving 5 g
of crude product which was recrystallized from methanol to give 2.2 g of 1-[2-
(2-carbethoxy-5,6-dimethoxy-3-indolyl)ethyl]-4-phenylpiperazine, M.P. 142.5-
144.0°C.
Therapeutic Function
Antipsychotic; Neuroleptic
Alpertine Preparation Products And Raw materials
Alpertine Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| MedChemexpress LLC | 021-58955995 | sales@medchemexpress.cn | United States | 4863 | 58 |
| Shenzhen Regent Biochemical Technology Co., Ltd. | 0755-0755-85201366 18938635012 | sales@regentsciences.com | China | 9288 | 58 |
| Shanghai Han-Xiang Chemical Co., Ltd. | 15971444841 | amber@biochempartner.com | China | 3063 | 58 |
| Beijing Jin Ming Biotechnology Co., Ltd. | 010-60605840 15801484223 | psaitong@jm-bio.com | China | 29834 | 58 |
| TargetMol Chemicals Inc. | 4008200310 | marketing@tsbiochem.com | China | 24131 | 58 |
27076-46-6(Alpertine)Related Search:
1of4