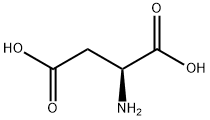
L-Aspartic acid
- CAS No.
- 56-84-8
- Chemical Name:
- L-Aspartic acid
- Synonyms
- ASPARTIC ACID;ASP;ASPARTATE;L-ASP;H-ASP-OH;Aspartic;Asparaginic acid;(S)-2-AMINOSUCCINIC ACID;L-Asp-OH;L-ASPARGINE
- CBNumber:
- CB3141599
- Molecular Formula:
- C4H7NO4
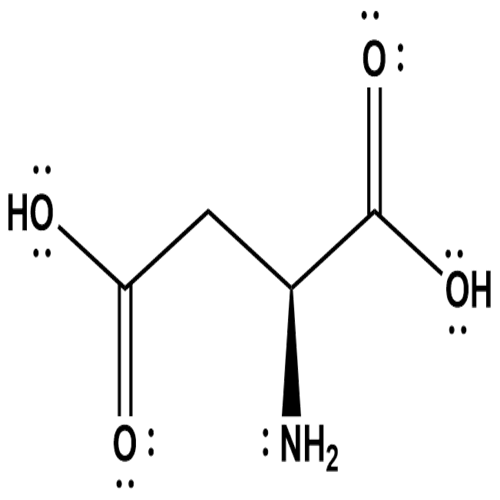
Lewis structure
- Molecular Weight:
- 133.1
- MDL Number:
- MFCD00002616
- MOL File:
- 56-84-8.mol
- MSDS File:
- SDS
| Melting point | >300 °C (dec.)(lit.) |
|---|---|
| alpha | 25 º (c=8, 6N HCl) |
| Boiling point | 245.59°C (rough estimate) |
| Density | 1.66 |
| refractive index | 1.4540 (estimate) |
| FEMA | 3656 | L-ASPARTIC ACID |
| storage temp. | Store below +30°C. |
| solubility | H2O: 5 mg/mL |
| form | powder |
| pka | 1.99(at 25℃) |
| color | White |
| PH | 2.5-3.5 (4g/l, H2O, 20℃) |
| Odor | acid taste |
| Odor Type | odorless |
| optical activity | [α]20/D +24.7±1°, c = 5% in 5 M HCl |
| Water Solubility | 5 g/L (25 ºC) |
| λmax |
λ: 260 nm Amax: 0.20 λ: 280 nm Amax: 0.10 |
| JECFA Number | 1429 |
| Merck | 14,840 |
| BRN | 1723530 |
| Stability | Stable. Combustible. Incompatible with strong oxidising agents. |
| InChIKey | CKLJMWTZIZZHCS-REOHCLBHSA-N |
| LogP | -0.67 |
| Substances Added to Food (formerly EAFUS) | L-ASPARTIC ACID |
| FDA 21 CFR | 172.320 |
| CAS DataBase Reference | 56-84-8(CAS DataBase Reference) |
| FDA UNII | 30KYC7MIAI |
| NIST Chemistry Reference | Aspartic acid(56-84-8) |
| EPA Substance Registry System | Aspartic acid (56-84-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P264-P270-P301+P312-P501 | |||||||||
| Hazard Codes | Xi,Xn | |||||||||
| Risk Statements | 36-36/37/38-20/21/22 | |||||||||
| Safety Statements | 26-24/25-22-36 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | CI9098500 | |||||||||
| F | 10 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | IRRITANT | |||||||||
| HS Code | 29224995 | |||||||||
| Toxicity | LD50 intraperitoneal in mouse: 6gm/kg | |||||||||
| NFPA 704 |
|
L-Aspartic acid price More Price(89)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHL80666 | L-Aspartic acid phyproof? Reference Substance | 56-84-8 | 100MG | $262 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.16003 | (S)-(+)-Aspartic acid forsynthesis | 56-84-8 | 100g | $54.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.16003 | (S)-(+)-Aspartic acid forsynthesis | 56-84-8 | 500g | $188 | 2024-03-01 | Buy |
| Sigma-Aldrich | 11189 | L-Aspartic acid BioUltra, ≥99.5% (T) | 56-84-8 | 1kg | $545 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1043819 | Aspartic acid United States Pharmacopeia (USP) Reference Standard | 56-84-8 | 100mg | $447 | 2024-03-01 | Buy |
L-Aspartic acid Chemical Properties,Uses,Production
Chemical Properties
The product is white crystal or crystalline powder, slightly sour in taste. Soluble in boiling water, slightly soluble in water at 25℃(0.5%), freely soluble in dilute acid and sodium hydroxide solution and insoluble in ethanol and ethyl ether. It decomposes when heated to 270 ℃. Its isoelectric point is 2.77 and its specific rotation is associated with the soluble solvent. It is dextral in the acid solution and water solution, but levorotary in the alkali solution. [α]25D+5.05 (C=0.5-2.0 g/ml, H2O). Combined with HNO2, alcohol or acyl chloride, it can produce L- malic acid, ester and amide respectively. It is the ingredient of unripe sugarcane and beet molasses.
Uses
It is used as an electrolyte supplement for aminophenol transfusion, inorganic ion supplement (K+, Ca+, etc.) and fatigue restorer. Potassium magnesium aspartate injection or oral solution can be used for arrhythmia, premature beat, tachycardia, hypokalemia, hypomagnesemia, heart failure, myocardial infarction, angina pectoris, hepatitis, cirrhosis and other diseases caused by cardiac glycoside poisoning. Due to its low toxicity, this product cannot be injected without dilution, and the patients with renal insufficiency and atrioventricular conduction block should use it with caution.
Production
L- aspartic acid is mainly produced by enzymatic method. L- aspartase acts on the fumaric acid and ammonia, that is, which generates L- aspartic acid.
- Strain Culturing: Eschrichia coli Asl.881 was cultured. The common meat juice medium is agarslantculture-medium. The vase medium comprises corn steep liquor 7.5%, fumaric acid 2.0% and MgSO4 7H2O 0.02%. Adjust the pH value of solution to 6.0 with ammonia water, then put 50-100ml culture medium into 500ml cone bottle after boiling and filtering. Take the fresh cultivated seeds on the slope or in the liquid, inoculate culture medium in shake flask, shake overnight at 37℃, adjust pH to 5.0 with 1mol/L HCl after enlarging culture step by step to 1000-2000L, cool it to room temperature after keeping 45℃ for 1h, centrifuge in rotary supercentrifuge and collect the thallus including aspartase.
- Immobilize aspartase: Make a bioreactor to take out 20kg E.Coli wet cell, suspend it in the culture supernatant after centrifugation in 80L (or 80L saline), keep it at 40℃ and then add 90L 12% gelatin solution and 1.0% glutaraldehyde solution, which should be held 40℃. Stir well, set aside to cool down and solidify, soak in 0.25% glutaraldehyde solution, hold 5℃ after an overnight, cut into small pieces ( 3-5 mm3) , soak in 0.25% glutaraldehyde solution at 5℃ for a night, take it out and elut with water, drain to obtain immobilized E.Coli containing aspartase and load it into filled bioreactor in reserve.
- Conversion: The solution of 1 mol/L of ammonium fumarate (including 1mmol/L MgCl2, pH8.5) substrate, which keeps 37℃, flows through a bioreactor at a constant speed (SV) continuously in the case of controlling the maximum conversion rate over 95% and then the conversion solution is obtained.
- Roughhew and refine: Add 1 mol/L of HCl into the conversion solution gradually to adjust pH valkue to 2.8, place at 5℃ overnight for crystallizing, filter to prepare crystallization, drain after water washing, drying at 105℃ to obtain L- aspartic acid crude. Use dilute ammonia to recrystallize, dissolve into 15% solution (pH5.0) with ammonia, add 1% activated carbon, stir and fade for 1h when heat to 70℃, filter immediately to remove slag, cool the filtrate, hold 5℃ overnight for crystallizing, filter to get crystallization and obtain the L-Aspartic acid finished products after vacuum drying at 85℃.
Description
L-Aspartic acid is the L-form of the aspartic acid. It is one of the 20 amino acids that used in the protein synthesis. It is the non-essential amino acids for humans as it can be synthesized in vivo. It is important in the synthesis of other amino acids and some nucleotides, and is a metabolite in the citric acid and urea cycles. In animals, it may be used as a neurotransmitter. It can be chemically synthesize from the diethyl sodium phthalimidomalonate. Currently, almost all the aspartic acids are manufactured in China. Its application include being used as low calorie sweetener (as the part of the aspartame), scale and corrosion inhibitor, and in resins. One of its growing applications is for the manufacturing of biodegradable superabsorbent polymer, polyaspartic acid. It can also be used in fertilizer industry to improve water retention and nitrogen uptake.
Chemical Properties
Apartic acid is an aliphatic monoaminodicarboxylic acid (amino acid) and is a well-known constituent of protein. It has a slight acid taste
Chemical Properties
Aspartic acid (abbreviated as Asp or D) is an α-amino acid with the chemical formula HOOCCH(NH2 )CH2COOH. The carboxylate anion, salt, or ester of aspartic acid is known as aspartate. The Lisomer of aspartate is one of the 20 proteinogenic amino acids, i.e., the building blocks of proteins. Its codons are GAU and GAC. Aspartic acid is, together with glutamic acid, classified as an acidic amino acid with a pKa of 3.9, however in a peptide the pKa is highly dependent on the local environment. A pKa as high as 14 is not at all uncommon. Aspartate is pervasive in biosynthesis. As with all amino acids, the presence of acid protons depends on the residue's local chemical environment and the pH of the solution.
Physical properties
Solubility 0.5 (25 ℃) g/100 g H2O, pI 2.98, dissociation constants: pK1 2.1, pK2 3.86 (β-COOH), pK3 9.82
Chemical Properties
Colorless crystals. Soluble in water; insoluble in alcohol and ether. Optically active. dl-aspartic acid.
Occurrence
Dietary sources
Aspartic acid is not an essential amino acid, which means that it can be synthesized from central metabolic pathway intermediates in humans. Aspartic acid is found in :
Animal sources : luncheon meats, sausage meat, wild game
Vegetable sources: sprouting seeds, oat flakes, avocado,
asparagus , young sugarcane, and molasses from sugar beets.
Chemical synthesis
Racemic aspartic acid can be synthesized from diethyl sodium phthalimido malonate, (C6H4(CO)2NC(CO2Et)2).
The major disadvantage of the above technique is that equimolar amounts of each enantiomer are made. Using biotechnology it is now possible to use immobilized enzymes to create just one type of enantiomer owing to their stereo specificity. Aspartic acid is made synthetically using ammonium fumarate and aspartase from E.coli, E.coli usually breaks down the aspartic acid as a nitrogen source but using excess amounts of ammonium fumarate a reversal of the enzyme's job is possible, and so aspartic acid is made to very high yields, 98.7 mM from 1 M.
History
Aspartic acid was first discovered in 1827 by Plisson, derived from asparagine, which had been isolated from asparagus juice in 1806, by boiling with a base.
Uses
l-aspartic acid is an amino acid used as a skin-conditioning agent.
Uses
L-Aspartic acid is used as a component of parenteral and enteral nutrition and as a pharmaceutical ingredient. it is used for cell culture and in manufacturing processes. It is widely utilized for mineral supplementation in the salt form.
Uses
L-aspartic acid is used as a dietary supplement, it can be blended with minerals to make compounds like potassium aspartate, copper aspartate, manganese aspartate, magnesium aspartate, zinc aspartate and more. Increasing the absorption, and hence utilization potentials, of these minerals via the addition of aspartate induces certain health benefits. Many athletes use L-aspartic acid-based mineral supplements orally to enhance their performance capacities. Aspartic acid and glutamic acid play important roles as general acids in enzyme active centers, as well as in maintaining the solubility and ionic character of proteins. It can help promote a robust metabolism, and is sometimes used to treat fatigue and depression. Aspartic acid is used as a component of parenteral and enteral nutrition. In pharmaceutical agents aspartic acid is used as an ammoniac detoxicating agent, hepar function accelerator and fatigue refresher.
Definition
ChEBI: The L-enantiomer of aspartic acid.
Aroma threshold values
Detection: 300 ppb
General Description
To request documentation for this product, please contact Customer Support and select ‘Product Documentation′. Please note that access to the documentation for this product requires a confidentiality disclosure agreement.
Hazard
Low toxicity.
Biological Activity
Endogenous NMDA receptor agonist.
Biochem/physiol Actions
Principal neurotransmitter for fast synaptic excitation.
Safety Profile
Low toxicity by intraperitoneal route. When heated to decomposition emits toxic fumes of NOx.
Synthesis
Aspartate is non - essential in mammals, being produced from oxaloacetate by transamination. It can also be generated from ornithine and citrulline in the urea cycle. In plants and microorganisms, aspartate is the precursor to several amino acids, including four that are essential for humans: methionine, threonine, isoleucine, and lysine. The conversion of aspartate to these other amino acids begins with reduction of aspartate to its "semi aldehyde," O2CCH(NH2)CH2CHO. Asparagine is derived from aspartate via trans amidation :
-O2CCH(NH2)CH2CO2 - + G C (O)NH3+ O2CCH(NH2)CH2CONH3+ + GC(O)O
(where GC(O)NH2 and GC(O)OH are glutamine and glutamic acid, respectively).
Synthesis
Enzymatically, aspartic acid is reversibly synthesized by a transamination reaction between oxaloacetic acid and glutamic acid in the presence of pyridoxal phosphate.
storage
Room temperature
Forms and nomenclature
There are two forms or enantiomers of aspartic acid. The name "aspartic acid" can refer to either enantiomer or a mixture of two. Of these two forms, only one, "L - aspartic acid", is directly incorporated into proteins. The biological roles of its counterpart, "Daspartic acid" are more limited. Where enzymatic synthesis will produce one or the other, most chemical syntheses will produce both forms, "DL-aspartic acid," known as a racemic mixture.
Other biochemical roles
Aspartate is also a metabolite in the urea cycle and participates in gluconeogenesis. It carries reducing equivalents in the malateaspartate shuttle, which utilizes the ready inter conversion of aspartate and oxaloacetate, which is the oxidized (dehydrogenated) derivative of malic acid. Aspartate donates one nitrogen atom in the biosynthesis of inosine, the precursor to the purine bases. In addition, aspartic acid acts as hydrogen acceptor in a chain of ATP synthase.
L-Aspartic acid Preparation Products And Raw materials
Raw materials
1of4
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| NINGBO CREATE-BIO ENGINEERING CO.,LTD | +8613906695486 | 13906695486@126.com | China | 1 | 58 |
| Shandong Juchuang Chemical Co., LTD | +86-18885615001 +86-18885615001 | admin@juchuangchem.com | China | 387 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-13474506593 +86-13474506593 | sarah@tnjone.com | China | 864 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 | admin@hbouhuang.com | China | 1175 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 | bj1@bj-chem.com | China | 298 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ | sales03@shyrchem.com | CHINA | 738 | 60 |
View Lastest Price from L-Aspartic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-20 | L-Aspartic Acid
56-84-8
|
US $0.00 / kg | 1kg | 99% | 10000kg | Shaanxi TNJONE Pharmaceutical Co., Ltd | |
 |
2024-04-18 | L-Aspartic acid
56-84-8
|
US $50.00 / kg | 1kg | 99.10% | 50000kg | Ouhuang Engineering Materials (Hubei) Co., Ltd | |
 |
2024-04-18 | L-Aspartic acid
56-84-8
|
US $30.00 / kg | 1kg | 99.7% | 200000kg | Ouhuang Engineering Materials (Hubei) Co., Ltd |
-

- L-Aspartic Acid
56-84-8
- US $0.00 / kg
- 99%
- Shaanxi TNJONE Pharmaceutical Co., Ltd
-

- L-Aspartic acid
56-84-8
- US $50.00 / kg
- 99.10%
- Ouhuang Engineering Materials (Hubei) Co., Ltd
-

- L-Aspartic acid
56-84-8
- US $30.00 / kg
- 99.7%
- Ouhuang Engineering Materials (Hubei) Co., Ltd