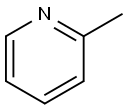
2-Picoline
- CAS No.
- 109-06-8
- Chemical Name:
- 2-Picoline
- Synonyms
- 2-METHYLPYRIDINE;α-picoline;o-Picoline;a-Picoline;2-Methylpyidine;pyridine,2-methyl-;ALPHAP;NSC-3409;ai3-2409;aPicolin
- CBNumber:
- CB3237870
- Molecular Formula:
- C6H7N
- Molecular Weight:
- 93.13
- MDL Number:
- MFCD00006332
- MOL File:
- 109-06-8.mol
- MSDS File:
- SDS
| Melting point | -70 °C (lit.) |
|---|---|
| Boiling point | 128-129 °C (lit.) |
| Density | 0.942-0.946 at 20 °C 0.943 g/mL at 25 °C (lit.) |
| vapor density | 3.2 (vs air) |
| vapor pressure | 10 mm Hg ( 24.4 °C) |
| refractive index |
n |
| Flash point | 79 °F |
| storage temp. | Store below +30°C. |
| solubility | H2O: freely soluble |
| form | Liquid |
| pka | 5.97(at 20℃) |
| color | Clear pale yellow |
| Odor | Unpleasant |
| PH | 8.5 (100g/l, H2O, 20℃) |
| explosive limit | 1.4-8.6%(V) |
| Odor Type | sweaty |
| Water Solubility | MISCIBLE |
| Sensitive | Hygroscopic |
| Merck | 14,7400 |
| BRN | 104581 |
| Dielectric constant | 9.8000000000000007 |
| Stability | Stable. Flammable. Incompatible with strong oxidizing agents, sulfuric acid, acids, metals, iron (II) sulfate, hydrogen peroxide. Hygroscopic. |
| InChIKey | BSKHPKMHTQYZBB-UHFFFAOYSA-N |
| LogP | 1.11 at 20℃ |
| CAS DataBase Reference | 109-06-8(CAS DataBase Reference) |
| EWG's Food Scores | 2 |
| FDA UNII | 3716Q16Q6A |
| NIST Chemistry Reference | Pyridine, 2-methyl-(109-06-8) |
| EPA Substance Registry System | 2-Methylpyridine (109-06-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H226-H302+H332-H311-H314-H335 | |||||||||
| Precautionary statements | P210-P280-P301+P312-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,T | |||||||||
| Risk Statements | 10-20/21/22-36/37-24-20/22 | |||||||||
| Safety Statements | 26-36-45-36/37 | |||||||||
| RIDADR | UN 2313 3/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | TJ4900000 | |||||||||
| Autoignition Temperature | 995 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29333999 | |||||||||
| Toxicity | LD50 orally in rats: 1.41 g/kg (Smyth) | |||||||||
| NFPA 704 |
|
2-Picoline price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 109835 | 2-Methylpyridine 98% | 109-06-8 | 25ml | $30.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 109835 | 2-Methylpyridine 98% | 109-06-8 | 1l | $112 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.09722 | 2-Methylpyridine for synthesis | 109-06-8 | 1L | $74.3 | 2022-05-15 | Buy |
| TCI Chemical | P0415 | 2-Methylpyridine >98.0%(GC)(T) | 109-06-8 | 25mL | $24 | 2024-03-01 | Buy |
| TCI Chemical | P0415 | 2-Methylpyridine >98.0%(GC)(T) | 109-06-8 | 500mL | $42 | 2024-03-01 | Buy |
2-Picoline Chemical Properties,Uses,Production
Chemical Properties
2-Methylpyridine is highly stable in aqueous solutions but decomposes when heated to emit NOx. The chemical also may react with oxidizing agents.
Chemical Properties
Picolines are colorless liquids. Strong, unpleasant, pyridine-like odor.“Picoline” is often used as mixed isomers.
Chemical Properties
colourless to yellow liquid with an unpleasant smell
Occurrence
2-Methylpyridine is released in atmospheric emissions from coal during processing into tar, pitch and coke (Windholz et al 1983; Naizer and Mashek 1974). It is also a byproduct of coal gasification and liquefaction processes (Pellizzori et al 1979; Stuermer et al 1982) and oil shale retorting (Pellizzari et al 1979). It is present in coal and is released in stack emissions (Opresko 1982). 2-Methylpyridine has been identified in effluences from the following industries: timber products, organic chemicals, pharmaceuticals and public waste treatment facilities (Schackleford and Cline 1983). 2-Methylpyridine also is a constituent of tobacco smoke (Brunneman 1978).
2-Methylpyridine is biodegradable. A 1 mM solution of 2-methylpyridine exposed in soil microorganism was completely degraded in 14-33 d under aerobic conditions, but not degraded after 97 d in anaerobic conditions (Naik et al 1972).
Uses
2-Picoline is used as a reagent in the synthesis of 2-Picolineborane, a non-toxic alternative to sodium borohydride for the labelling of oligosaccharides.
Uses
2-Picoline is used as an intermediate in agrochemicals and pharmaceuticals. It serves as a solvent as well as to prepare dyes and resins. It finds application as a constituent in cigarette smoke, bone oil, coal tar and coke oven emissions. Further, it acts as a precursor of 2-vinylpyridine, picolinic acid and nitrapyrin. It is also employed to study the electron and proton transfer reactions of lumiflavin. In addition, it is used in the synthetic pathway for the preparation of dearomatized, allylated and carbon-hydrogen bond activated pyridine derivatives.
Uses
Solvent; intermediate in the dye and resins industries.
Definition
ChEBI: 2-methylpyridine is a methylpyridine carrying a methyl substituent at position 2.
Production Methods
2-Methylpyridine is synthesized by distillation of coal tar or bone oil or by vapor phase reaction of acetaldehyde and ammonia in a 3:1 ratio followed by isolation of 2-methylpyridine from the reaction mixture (Considine 1974). It also can be synthesized from cyclohexylamine with excess ammonia and ZnCl2 at 350°C, resulting in a 40-50% yield; or prepared from ethylene-mercuric acetate adduct with ammonia water with a 70% yield (Windholz et al 1983). Production in 1977 probably exceeded one million pounds (Opresko 1982).
Synthesis Reference(s)
Journal of the American Chemical Society, 86, p. 5355, 1964 DOI: 10.1021/ja01077a077
Synthesis, p. 26, 1976
Tetrahedron Letters, 17, p. 383, 1976 DOI: 10.1016/S0040-4039(00)93738-9
General Description
Colorless liquid with a strong, unpleasant odor. Floats on water. Poisonous vapor is produced.
Air & Water Reactions
Highly flammable. Water soluble.
Reactivity Profile
2-Picoline is hygroscopic. 2-Picoline reacts with hydrogen peroxide, iron(II) sulfate, sulfuric acid, oxidizing agents, acids, and metals.
Health Hazard
INHALATION, INGESTION OR SKIN ABSORPTION: Narcosis, headache, nausea, giddiness, vomiting. EYES: Severe irritation. SKIN: Causes burns. INGESTION: Irritation and gastric upset.
Health Hazard
2-Methylpyridine causes local irritation on contact with the skin, mucous membranes and cornea (Reinhardt and Brittelli 1981). Clinical signs of intoxication caused by the methyl pyridines include weight loss, diarrhea, weakness, ataxia and unconsciousness (Reinhardt and Brittelli 1981) as well as narcosis headache, nausea, giddiness and vomiting (Ketchen and Porter 1979). Chronic exposure to methylpyridine results in anemia and ocular and facial paralysis in addition to the previously mentioned symptoms (Ketchen and Porter 1979).
Flammability and Explosibility
Flammable
Industrial uses
2-Methylpyridine is used as a solvent, or as a chemical intermediate in the dye and resin industries (Windholz et al 1983) or for pharmaceuticals and rubber (Hawley 1981). It is used to make 2-vinylpyridine which is in turn made into a terpolymer with styrene and butadiene. The latexes of these terpolymers are extensively employed in adhesives for bonding textiles to elastomers (Reinhart and Britelli 1981). It is also a chemical intermediate for 2-chloro-6-(trichloromethyl)pyridine and 2-vinylpyridine.
Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion and skin contact. Mildly toxic by inhalation. A skin and severe eye irritant. Mutation data reported. Flammable liquid when exposed to heat or flame. To fight fire, use CO2, dry chemical. Mixtures with hydrogen peroxide + iron(II) sulfate + sulfuric acid may igmte and then explode. When heated to decomposition it emits toxic fumes of NOx.
Potential Exposure
(o-isomer); Suspected reprotoxic hazard, Primary irritant (w/o allergic reaction), (m-isomer): Possible risk of forming tumors, Primary irritant (w/o allergic reaction). Picolines are used as intermediates in pharmaceutical manufacture, pesticide manufacture; and in the manufacture of dyes and rubber chemicals. It is also used as a solvent.
Carcinogenicity
No reliable studies in mammals to evaluate the carcinogenic potential of any of the three methylpyridines were found. None of the methylpyridines is listed as a carcinogen by IARC, NTP, OSHA, or ACGIH.
Metabolism
Methylpyridines are absorbed by inhalation, ingestion or percutaneous absorption (Parmeggiana 1983). 2-Methylpyridine was rapidly absorbed and penetrated to the liver, heart, spleen, lungs and muscle during the first 10-20 min following oral administration of 0.5 g/kg to rats (Kupor 1972). The percentage uptake of 2-methylpyridine by rats increased with dosage and its elimination occurred in two phases which also were dose dependent (Zharikov and Titov 1982).
Data on the biotransformation of 2-methylpyridine have been summarized by Williams (1959) and DeBruin (1976). In rabbits and dogs, the compound is oxidized to α-picolinic acid and then conjugated with glycine to form α-picolinuric acid which is excreted in the urine. In hens, it is excreted partially as α-pyridinornithuric acid. About 96% of a 100 mg/kg oral dose of 2-methylpyridine in rats was excreted in the urine as picolinuric acid (Hawksworth and Scheline 1975). There also is evidence that 2-methylpyridine forms an 2-methylated derivative in dogs (Williams 1959). Since 3-methylpyridine is converted to its N-oxide in various species (Gorrod and Damani 1980), it is likely that 2-methyl-pyridine also is similarly oxidized.
Shipping
UN2313 Picolines, Hazard Class: 3; Labels: 3-Flammable liquid.
Purification Methods
Biddiscombe and Handley [J Chem Soc 1957 1954] steam distilled a boiling solution of the base in 1.2 equivalents of 20% H2SO4 until about 10% of the base had been carried over, along with non-basic impurities. Excess aqueous NaOH is then added to the residue, the free base is separated, dried with solid NaOH and fractionally distilled. 2-Methylpyridine can also be dried with BaO, CaO, CaH2, LiAlH4, sodium or Linde type 5A molecular sieves. An alternative purification is via the ZnCl2 adduct, which is formed by adding 2-methylpyridine (90mL) to a solution of anhydrous ZnCl2 (168g) and 42mL conc HCl in absolute EtOH (200mL). Crystals of the complex are filtered off, recrystallised twice from absolute EtOH (to give m 118.5-119.5o), and the free base is liberated by addition of excess aqueous NaOH. It is steam distilled, and solid NaOH is added to the distillate to form two layers, the upper one of which is then dried with KOH pellets, stored for several days with BaO and fractionally distilled. Instead of ZnCl2, HgCl2 (430g in 2.4L of hot water) can be used. The complex, which separates on cooling, can be dried at 110o and recrystallised from 1% HCl (to m 156-157o). The hydrochloride has m 78-79o, and the picrate has m 165.5o(from EtOH) and 180o(from H2O). [Beilstein 20 III/IV 2679, 20/5 V 464.]
Incompatibilities
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Attacks copper and its alloys.
2-Picoline Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of6
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15371 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
Related articles
- 2-Methylpyridine: Properties, Production process and Uses
- 2-Methylpyridine can be used to synthesize an important polymer monomer, 2-vinylpyridine, which is widely used in the rubber i....
- Mar 25,2024
- What is 2-Methylpyridine?
- 2-Methylpyridine, or 2-picoline, is the compound described with formula C6H7N. 2-Picoline is a colorless liquid that has an un....
- Jul 22,2021
View Lastest Price from 2-Picoline manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-10-07 | 2-Picoline
109-06-8
|
US $10.00-1.00 / kg | 1kg | 0.99 | 20 tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-09-06 | 2-Picoline
109-06-8
|
US $0.00 / KG | 1KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. | |
 |
2023-08-29 | 2-Picoline
109-06-8
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd |
-

- 2-Picoline
109-06-8
- US $10.00-1.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.
-

- 2-Picoline
109-06-8
- US $0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
-

- 2-Picoline
109-06-8
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd