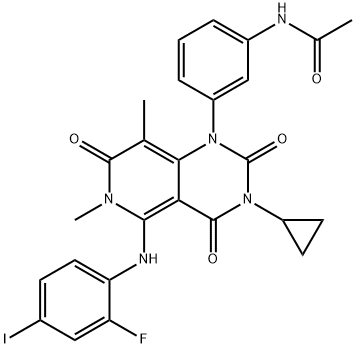
Trametinib
- CAS No.
- 871700-17-3
- Chemical Name:
- Trametinib
- Synonyms
- GSK-1120212;TraMetinib API;Mekinist trametinib;Trametinib free base;N-(3-{3-Cyclopropyl-5-[(2-fluoro-4-iodophenyl)aMino]-6,8-diMethyl-2,4,7-trioxo-3,4,6,7-tetrahydropyrido[4,3-d]pyriMidin-1(2H)-yl}phenyl)acetaMide;CS-30;100836;Mekinist);TraMetinib;QuMei iMatinib
- CBNumber:
- CB32514557
- Molecular Formula:
- C26H23FIN5O4
- Molecular Weight:
- 615.39
- MDL Number:
- MFCD17215075
- MOL File:
- 871700-17-3.mol
| Melting point | 300-301 °C |
|---|---|
| Density | 1.74 |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 20 mg/ml) |
| form | White solid. |
| pka | 14.76±0.70(Predicted) |
| color | White |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 2 months. |
| FDA UNII | 33E86K87QN |
| ATC code | L01EE01 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H317-H361-H372 |
| Precautionary statements | P261-P272-P280-P302+P352-P333+P313-P321-P363-P501-P201-P202-P281-P308+P313-P405-P501-P260-P264-P270-P314-P501 |
| Safety Statements | 24/25 |
| HS Code | 29339900 |
Trametinib price More Price(52)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 16292 | Trametinib ≥95% | 871700-17-3 | 25mg | $68 | 2024-03-01 | Buy |
| Cayman Chemical | 16292 | Trametinib ≥95% | 871700-17-3 | 50mg | $116 | 2024-03-01 | Buy |
| Cayman Chemical | 16292 | Trametinib ≥95% | 871700-17-3 | 100mg | $135 | 2024-03-01 | Buy |
| Cayman Chemical | 16292 | Trametinib ≥95% | 871700-17-3 | 250mg | $302 | 2024-03-01 | Buy |
| TRC | C988930 | Trametinib | 871700-17-3 | 200mg | $250 | 2021-12-16 | Buy |
Trametinib Chemical Properties,Uses,Production
Abstract
Trametinib (trade name Mekinist) is a cancer drug. It is a MEK inhibitor drug with anti-cancer activity.
Trametinib(Mekinist) is a reversible, highly selective, allosteric inhibitor of mitogen-activated extracellular signal regulated kinase 1 (MEK1) and MEK2 activation and kinase activity. MEK proteins are components of the extracellular signal-related kinase (ERK) pathway. In melanoma and other cancers, this pathway is often activated by mutated forms of BRAF which activates MEK. Trametinib inhibits activation of MEK by BRAF and inhibits MEK kinase activity. Trametinib inhibits growth of BRAF V600 mutant melanoma cell lines and demonstrates anti-tumor effects in BRAF V600 mutant melanoma animal models.
Mekinist is indicated as monotherapy or in combination with Tafinlar (dabrafenib) for the treatment of adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation.
Biological activity
Trametinib (GSK1120212) is a highly specific and potent MEK1/2 inhibitor with IC50 of 0.92 nM/1.8 nM in cell-free assays, no inhibition of the kinase activities of c-Raf, B-Raf, ERK1/2.
In vitro
For the different subtypes of Raf and MEK, with IC50 ranging from 0.92 nM to 3.4 nM,GSK1120212 inhibits the phosphorylation of MBP. GSK1120212 demonstrates no inhibition of the kinase activities of c-Raf, B-Raf, ERK1 and ERK2. In addition, GSK1120212 does not show drastic inhibitory activity against the other 98 kinases. GSK1120212 displays potent inhibitory activity against human colorectal cancer cell lines. HT-29 and COLO205 cells, which are known to have a constitutively active B-Raf mutant, are most sensitive to GSK1120212 with IC50 0.48 nM and 0.52 nM, respectively. The cell lines bearing a K-Ras mutation show a wide range of sensitivity to GSK1120212 with IC50 of 2.2-174 nM. In contrast, COLO320 DM cells, bearing the wild-type gene in both B-Raf and K-Ras, are found to be resistant to GSK1120212 even at 10 μM. GSK1120212 treatment for 24 hours induces cell-cycle arrest at the G1 phase in all sensitive cell lines. Consistently, GSK1120212 treatment leads to upregulation of p15INK4b and/or p27KIP1 in most of the colorectal cancer cell lines. GSK1120212 induces apoptosis both in HT-29 and COLO205 cells, but that COLO205 cells are more sensitive to GSK1120212 than HT-29 cells in terms of apoptosis induction.GSK1120212 blocks tumor necrosis factor-α and interleukin-6 production from peripheral blood mononuclear cells (PBMCs).
In vivo
Oral administration of GSK1120212 at 0.3 mg/kg or 1 mg/kg once daily for 14 days is effective in inhibiting the HT-29 xenograft growth, and 1 mg/kg of GSK1120212 can completely block the tumor increase. The phosphorylation of ERK1/2 is completely inhibited in the established tumor tissues by single oral dose of 1 mg/kg GSK1120212, and both p15INK4b and p27KIP1 protein levels are upregulated after 14 days of treatment with GSK1120212. In the COLO205 xenograft model, tumor regression is observed even at a dose of 0.3 mg/kg. At a dose of 1 mg/kg, a complete regression is obtained in 4 out of 6 mice in which the tumor degenerates to the point that tumor volume is not measurable. Administration of GSK1120212 at 0.1 mg/kg almost completely suppresses adjuvant-induced arthritis (AIA) and type II collagen-induced arthritis (CIA) in Lewis rats or DBA1/J mice, respectively.
Features
More potent than PD0325901 or AZD6244.
References
https://en.wikipedia.org/wiki/Trametinib
https://www.novartisoncology.com/news/product-portfolio/mekinist
Description
Trametinib is an inhibitor of MEK1 and -2. It inhibits B-RAF- and C-RAF-induced phosphorylation of MEK1 (IC50s = 3.4 and 1.8 nM, respectively) and MEK2 (IC50s = 1.6 and 0.92 nM, respectively). Trametinib inhibits the growth of two human colorectal cancer cell lines expressing mutant B-RAF (IC50s = 0.48 and 0.52 nM) and seven cell lines expressing mutant K-Ras (IC50s = 2.2-174 nM) but does not inhibit the growth of wild-type COLO 320DM cells expressing both B-RAF and K-Ras (IC50 = >10,000 nM). It reduces tumor growth in HT-29 and COLO 205 mouse xenograft models when used at doses of 0.3 and 1 mg/kg per day. Trametinib (0.03 and 0.1 mg/kg per day) also decreases M. tuberculosis-induced increases in hind paw volume in a rat model of arthritis. Formulations containing trametinib, in combination with dabrafenib, have been used in the treatment of metastatic mutant B-RAFV600E melanoma.
Uses
Used in the systematic treatment of advanced cutaneous melanoma. An EGFR kinase inhibitor and potent MEK inhibitor.
Uses
antiprotozoal
Uses
Used in the systematic treatment of advanced cutaneous melanoma. An EGFR kinase inhibitor.
Indications
MEK, also known as MAPK, is a dual specificity threonine/tyrosine kinase that is a key node in the Raf–Ras–MEK signaling pathway. Small-molecule MEK inhibitors represent the largest group of type III allosteric inhibitors that do not bind to the ATP binding pocket. As of December 2016, besides the FDA-approved MEK1/2 inhibitors trametinib (Mekinist(R), GlaxoSmithKline) and cobimetinib (Cotellic(R), Roche), over 10 MEK inhibitors are currently in clinical trials. Trametinib was approved by FDA in 2013 for the treatment of patients with either B-Raf V600E or V600K mutated metastatic melanoma. Considering the fact that MEK and Raf are different kinases along the same pathway of Ras–Raf–MEK/ERK signaling cascade, combination strategies using both MEK and B-Raf inhibitors were utilized to overcome the observed progression using single-agent trametinib, which usually occurs within 7months. FDA approved the combination of trametinib and dabrafenib for the treatment of B-Raf V600E/K mutated metastatic melanoma in January 2014 and the combination of cobimetinib and vemurafenib for the same type of indication. Although significant improvement in progression-free survival was observed using MEK/B-Raf combination strategy, the incidence of some common adverse effects, such as vomiting, diarrhea, nausea, rash, and pyrexia, also increased.
Definition
ChEBI: A pyridopyrimidine that is used (as its dimethyl sulfoxide addition compound) for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations, and who have not received prior BRAF inhibitor treatment.
Clinical Use
Trametinib (GSK1120212) is an oral MEK inhibitor which has demonstrated excellent results in combination therapy for BRAF-mutated melanoma and is FDA approved in combination with BRAF inhibitors for that indication. Trametinib was evaluated initially as a single agent in KRAS mutant NSCLC in comparison with docetaxel and pemetrexed. Results as a single agent were not impressive, with an ORR of only 12% in these patients.
target
MEK1
Drug interactions
Potentially hazardous interactions with other drugs
Antipsychotics: avoid with clozapine - increased risk
of agranulocytosis.
Metabolism
Metabolised mainly by deacetylation alone or in
combination with mono-oxygenation. The deacetylated
metabolite was further metabolised by glucuronidation.
CYP3A4 oxidation is considered a minor pathway
of metabolism. The deacetylation is mediated by
the carboxyl-esterases 1b, 1c and 2, with possible
contributions by other hydrolytic enzymes.
Total dose recovery was low after a 10-day collection
period (<50
%) following administration of a single
oral dose of radiolabelled trametinib, due to the long
elimination
Half-life. Trametinib was excreted mainly
in the faeces (>80
% of recovered radioactivity) and to a
minor extent in urine (≤19
%).
storage
Store at -20°C
References
1) Gilmartin?et al.?(2011),?GSK1120212 (JTP-74057) is an Inhibitor of MEK Activity and Activation with Favorable Pharmacokinetic Properties for Sustained In Vivo Pathway Inhibition; Clin. Cancer Res.?17?989 2) Abe?et al. (2011),?Discovery of a Highly Potent and Selective MEK Inhibitor: GSK1120212 (JTP-74057 DMSO solvate); ACS Med. Chem. Lett.?2?320 3) Allegrezza?et al.?(2016),?Trametinib Drives T-cell-Dependent Control of KRAS Tumors by Inhibiting Pathological Myelopoiesis; Cancer Res.?76?6253 4) Vella?et al.?(2014),?MEK inhibition, alone or in combination with BRAF inhibition, affects multiple functions of isolated normal human lymphocytes and dendritic cells; Cancer Immunol. Res.?2?351 5) Yamaguchi?et al.?(2012),?Suppressive effect of an orally active MEK1/2 inhibitor in two different animal models for rheumatoid arthritis: a comparison with leflunomide; Inflamm. Res.?61?445
Trametinib Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| BIONNA MEDICINE CO.,LTD | 01056380788-8515; +8618518759099 | 790226113@qq.com | China | 52 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
| Nanjing Finetech Chemical Co., Ltd. | 025-85710122 17714198479 | sales@fine-chemtech.com | CHINA | 885 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Biochempartner | 0086-13720134139 | candy@biochempartner.com | CHINA | 967 | 58 |
| HubeiwidelychemicaltechnologyCo.,Ltd | 18627774460 | faith@widelychemical.com | CHINA | 742 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
Related articles
- What diseases are treated with the combination of dabrafenib and trametinib?
- Dabrafenib and trametinib are targeted drugs that can be used together to treat melanoma and non-small cell lung cancer.
- Mar 21,2024
- Trametinib: Potent MEK Inhibitor with Specific Pharmacokinetic and Pharmacodynamic Properties
- Trametinib has specific pharmacokinetic properties, high plasma protein binding, and limited drug interactions. It effectively....
- Feb 20,2024
- Trametinib effects and side effects
- Trametinib is in a class of medications called kinase inhibitors. It works by blocking the action of an abnormal protein that ....
- Dec 23,2022
View Lastest Price from Trametinib manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-03-06 | Trametinib
871700-17-3
|
US $10.00 / Kg/Drum | 1KG | 98% | 10 ton | Hebei Guanlang Biotechnology Co,.LTD | |
 |
2023-01-31 | Trametinib
871700-17-3
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2021-10-20 | Trametinib
871700-17-3
|
US $95.00-79.00 / KG | 1KG | 99% | 9000kg/per week | Hebei Lingding Biotechnology Co., Ltd. |
-

- Trametinib
871700-17-3
- US $10.00 / Kg/Drum
- 98%
- Hebei Guanlang Biotechnology Co,.LTD
-

- Trametinib
871700-17-3
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Trametinib
871700-17-3
- US $95.00-79.00 / KG
- 99%
- Hebei Lingding Biotechnology Co., Ltd.