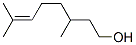CITRONELLOL
- CAS No.
- 26489-01-0
- Chemical Name:
- CITRONELLOL
- Synonyms
- FEMA 2307;FEMA 2309;CITRONELLOL AJ;CITRONELLOL 96;CITRONELLOL 80;DL-CITRONELLOL;DL-B-CITRONELLOL;CITRONELLOL PRIME;CITRONELLOL EXTRA;CITRONELLOL 90/92
- CBNumber:
- CB3377987
- Molecular Formula:
- C10H20O
- Molecular Weight:
- 156.27
- MDL Number:
- MFCD00002935
- MOL File:
- 26489-01-0.mol
| Melting point | 77-83 °C(lit.) |
|---|---|
| Boiling point | 225 °C(lit.) |
| Density | 0.857 g/mL at 25 °C(lit.) |
| vapor density | 5.4 (vs air) |
| vapor pressure | ~0.02 mm Hg ( 25 °C) |
| refractive index |
n |
| Flash point | 209 °F |
| storage temp. | 2-8°C |
| CAS DataBase Reference | 26489-01-0(CAS DataBase Reference) |
CITRONELLOL Chemical Properties,Uses,Production
Chemical Properties
The enantiomers (3R)-(+)-citronellol and (3S)-(?)-citronellol
occur in many essential oils.
(?)-Citronellol isolated from natural sources is often named rhodinol. At
present, the name rhodinol is also used for the isopropenyl isomer α-citronellol
or a mixture of the two isomers.
In many natural products, citronellol occurs as a mixture of its two enantiomers;
the pure (+) or (?) formis seldom found. (?)-Citronellol is the predominant enantiomer
in geranium and rose oils, both of whichmay contain up to 50% citronellols.
Citronellol is a colorless liquid with a sweet rose-like odor. The odor of (?)-
citronellol is more delicate than that of (+)-citronellol.
Citronellol undergoes the typical reactions of primary alcohols. Compared with
geraniol, which contains one more double bond, citronellol is relatively stable.
Citronellol is converted into citronellal by dehydrogenation or oxidation; hydrogenation
yields 3,7-dimethyloctan-l-ol. Citronellyl esters are easily prepared by
esterification with acid anhydrides.
Preparation
(?)-Citronellol is still obtained mainly from geranium oil by
saponification followed by fractional distillation (“rhodinol”). Although of high
odor quality, this grade does not possess the true (?)-citronellol odor due to
impurities. Much larger quantities of (+)-citronellol and racemic citronellol are
used and are prepared by partial or total synthesis.
1) Synthesis from citronellal: Citronellal can be hydrogenated to citronellol
by the use of special catalysts and/or special hydrogenation techniques,
for example, [122]. The citronellal that is used as the starting material
may originate from synthetic production or from isolation of essential
oils. Citronellal from citronella oil yields (+)-citronellol; the corresponding
material from citronellal from Eucalyptus citriodora oil is racemic. Pure
(+)-citronellol is also obtained from (+)-citronellal, which is produced as an
intermediate of (?)-menthol. By this asymmetric technology,
pure (?)-citronellal and, therefore, pure (?)-citronellol are also available.
2) Synthesis of racemic or slightly dextrorotatory citronellol from geraniol
fractions of essential oils: This citronellol is produced by catalytic hydrogenation of saponified geraniol fractions (also containing (+)-
citronellol) obtained from Java citronella oil, followed by fractional
distillation. Selective hydrogenation of the double bond in the 2-position
of geraniol in geraniol–citronellol mixtures isolated from essential oils
can be achieved by using Raney cobalt as a catalyst; overhydrogenation to
3,7-dimethyloctan-l-ol can be largely avoided by this method.
3) Synthesis of racemic citronellol from synthetic geraniol, nerol, or citral: A considerable
amount of commercial synthetic racemic citronellol is produced by
partial hydrogenation of synthetic geraniol and/or nerol. Another starting
material is citral, which can be hydrogenated, for example, in the presence
of a catalyst system consisting of transition metals and amines.
4) Preparation of (?)-citronellol from optically active pinenes: (+)-cis-Pinane is
readily synthesized by hydrogenation of (+)-α-pinene or (+)-β-pinene and is
then pyrolyzed to give (+)-3,7-dimethyl-1,6-octadiene. This compound can
be converted into (?)-citronellol (97% purity) by reaction with triisobutylaluminumor
diisobutylaluminumhydride, followed by air oxidation and hydrolysis
of the resulting aluminum alcoholate.
Definition
ChEBI: Citronellol is a monoterpenoid that is oct-6-ene substituted by a hydroxy group at position 1 and methyl groups at positions 3 and 7. It has a role as a plant metabolite.
Contact allergens
L-Citronellol is a constituent of rose and geranium oils. d-Citronellol occurs in Ceylon and Java citronella oils. As a fragrance allergen, citronellol has to be mentioned by name in cosmetics within the EU.
CITRONELLOL Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; | laboratory@coreychem.com | China | 30255 | 58 |
| ZHEJIANG JIUZHOU CHEM CO., LTD | +86-0576225566889 +86-13454675544 | admin@jiuzhou-chem.com;jamie@jiuzhou-chem.com;alice@jiuzhou-chem.com | China | 20000 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| 3B Pharmachem (Wuhan) International Co.,Ltd. | 821-50328103-801 18930552037 | 3bsc@sina.com | China | 15848 | 69 |
| Zhengzhou Acme Chemical Co., Ltd. | 0371-037163312495,13303845143 13303845143 | 3001379618@qq.com | China | 9939 | 58 |
| Hebei Zhenjia new material Co., LTD | 0319-5925599 13315915972 | 13313090628@163.com | China | 3489 | 58 |