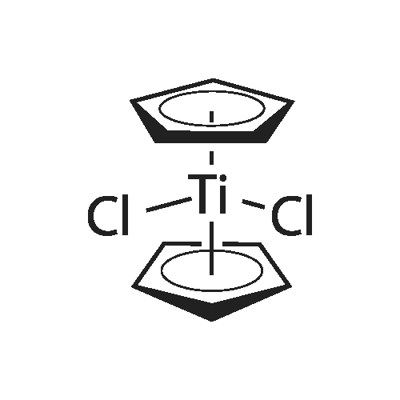
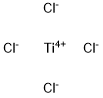
Titanocene dichloride
- CAS No.
- 1271-19-8
- Chemical Name:
- Titanocene dichloride
- Synonyms
- Cp2TiCl2;BIS(CYCLOPENTADIENYL)TITANIUM DICHLORIDE;TDC;Dichlorotitanocene;BIS(CYCLOPENTADIENYL)TITANIUM(IV) DICHLORIDE;NCI-C04502;titanium(2+);)titanium dichL;titaniumferrocene;Titanocene dichlorid
- CBNumber:
- CB3458745
- Molecular Formula:
- C10H10Cl2Ti
- Molecular Weight:
- 248.96
- MDL Number:
- MFCD00003723
- MOL File:
- 1271-19-8.mol
- MSDS File:
- SDS
| Melting point | 260-280 °C (dec.)(lit.) |
|---|---|
| Boiling point | 355.52°C (estimate) |
| Density | 1.6 g/mL at 25 °C(lit.) |
| vapor pressure | 0.002Pa at 25℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | slightly soluble in H2O, benzene; soluble in chloroform,ethanol, toluene |
| form | crystal |
| color | red |
| Specific Gravity | 1.6 |
| Water Solubility | slow decomposition |
| Sensitive | Air & Moisture Sensitive |
| Hydrolytic Sensitivity | 4: no reaction with water under neutral conditions |
| Merck | 14,9482 |
| Stability | Stable. Incompatible with strong oxidizing agents. Decomposes in water. Moisture sensitive. |
| LogP | -1.35 at 20℃ |
| CAS DataBase Reference | 1271-19-8(CAS DataBase Reference) |
| FDA UNII | MJE0547U1U |
| NIST Chemistry Reference | Titanocene dichloride(1271-19-8) |
| EPA Substance Registry System | Titanocene dichloride (1271-19-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi,T | |||||||||
| Risk Statements | 37/38-61-40-34-33-36/37/38 | |||||||||
| Safety Statements | 36-7/8-6A-45-36/37/39-26 | |||||||||
| RIDADR | UN3261 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | XR2050000 | |||||||||
| F | 8-10-21 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29310095 | |||||||||
| Toxicity | Titanocene dichloride may cause skin and mucous membrane irritation. The LD50 in mice has been reported as 60 mg/kg (i.p.) and 180 mg/kg (i.v.). In the rat, the LD50 is 25 mg/kg (i.p.). | |||||||||
| NFPA 704 |
|
Titanocene dichloride price More Price(50)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 234826 | Bis(cyclopentadienyl)titanium(IV) dichloride 97% | 1271-19-8 | 10g | $55.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 234826 | Bis(cyclopentadienyl)titanium(IV) dichloride 97% | 1271-19-8 | 50g | $181 | 2024-03-01 | Buy |
| TCI Chemical | T0616 | Titanocene Dichloride >98.0%(T) | 1271-19-8 | 5g | $26 | 2024-03-01 | Buy |
| TCI Chemical | T0616 | Titanocene Dichloride >98.0%(T) | 1271-19-8 | 25g | $62 | 2024-03-01 | Buy |
| Alfa Aesar | 040386 | Bis(cyclopentadienyl)titanium dichloride, 99+% | 1271-19-8 | 2g | $20.65 | 2024-03-01 | Buy |
Titanocene dichloride Chemical Properties,Uses,Production
Description
Titanocene dichloride is an organotitanium compound with the chemical formula (η5-C5H5)2TiCl2, often written as Cp2TiCl2, which is a common reagent in organometallic chemistry and organic synthesis. Cp2TiCl2 does not form a "sandwich" structure like ferrocene, but a tetrahedral structure due to its four ligands around a metal center. Because of its anti-tumor activity, it has been used in clinical trials as a chemotherapeutic agent.
Chemical Properties
Titanocene dichloride is a reddish-orange crystalline solid. Moderately soluble in toluene, chloroform, alcohol, and other hydroxylic solvents; sparingly soluble in water, petroleum ether, benzene, ether, carbon disulfide, and carbon tetrachloride. Stable in dry air, slowly hydrolyzed in moist air. Titanocene dichloride is irritating to the skin and mucous membranes.
Uses
Titanocene dichloride is used as an experimental cancer chemotherapeutic agent. It is used as a research chemical, as a catalyst in Ziegler–Natta polymerization reactions, and as an implant material in orthopedics, oral surgery, and neurosurgery.This metallocene is a common reagent in organometallic and organic synthesis. Titanocene dichloride is used to prepare titanocene pentasulfide. Used as an anticancer drug.
Preparation
Cp2TiCl2 continues to be prepared similarly to its original synthesis by Wilkinson and Birmingham:
2 NaC5H5 + TiCl4 → (C5H5)2TiCl2 + 2 NaCl
The reaction is conducted in THF. Work-up entails extraction into chloroform/hydrogen chloride and recrystallization from toluene. In the original literature, the structure was poorly understood. Each of the two Cp rings are attached to Ti(IV) through all five carbon atoms. In organometallic chemical jargon, this bonding is referred to as η5 (see hapticity).
Production Methods
Titanocene dichloride is produced by the reaction of titanium tetrachloride with cyclopentadienyl sodium.
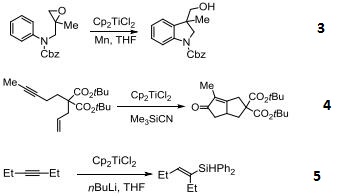
Reactions
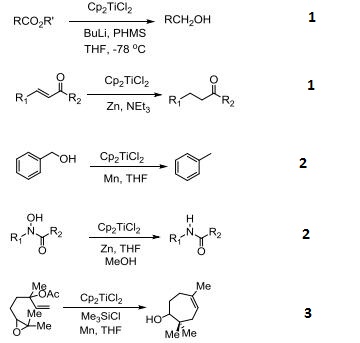
- Precatalyst for reduction of esters and |á,|?-unsaturated ketones.
- Catalyzes reductive deoxygenation of alcohols and hydroxylamines.
- Catalyst for the radical cyclization of epoxides.
- Reagent for the conversion of enynes to bicyclic cyclopentenones.
- Catalyzes silylation of alkenes and alkynes.
General Description
Titanocene dichloride appears as red to red-orange crystals. (NTP, 1992)
Air & Water Reactions
Stable in dry air. Decomposes in moist air and in water to form HCl .
Reactivity Profile
Titanocene dichloride is incompatible with strong oxidizers. Titanocene dichloride may decompose on exposure to water. .
Hazard
Toxic by inhalation, irritant to skin and mucous membranes.
Fire Hazard
Flash point data for Titanocene dichloride are not available; however, Titanocene dichloride is probably combustible.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by intravenous and intraperitoneal routes. Questionable carcinogen with experimental neoplastigenic, tumorigenic, and teratogenic data. Mutation data reported. See also TITANIUM COMPOUNDS. When heated to decomposition it emits toxic fumes of Cl-
Carcinogenicity
Based on the results of 2 year gavage studies, the National Toxicology Program determined that there was equivocal evidence of the carcinogenic activity of titanocene dichloride in male and female rats based on a marginal increase in the incidence of forestomach squamous cell effects.
Purification Methods
It forms bright red crystals from toluene or xylene/CHCl3 (1:1) and sublimes at 190o/2mm. It is moderately soluble in EtOH and insoluble in Et2O, *C6H6, CS2, CCl4, pet ether and H2O. The crystalline dipicrate explodes on melting at 139-140o. [Wilkinson et al. J Am Chem Soc 75 1011 1953, IR: Wilkinson & Birmingham J Am Chem Soc 76 4281 1954, NMR and X-ray: Glivicky & McCowan Can J Chem 51 2609 1973, Clearfield et al. J Am Chem Soc 53 1622 1975, Beilstein 16 IV 1769.]
Clinical claims and research
Titanocene dichloride, [Ti(Z5 -C5H5)2Cl2], was the first organometallic transition metal compound to be investigated clinically as an anticancer agent. It contains a cis-dichloride motif as cisplatin and was selected from several early transition metal cyclopentadienyl complexes as the best candidate for further development. The chemistry of the hard Ti(IV) ion is different from that of Pt(II): for example, cisplatin binds preferentially to the N7 of guanine in DNA, whereas titanocene dichloride exhibits higher affinity for the phosphate backbone. [Ti(Z5 -C5H5)2Cl2] hydrolyzes quickly in water, yielding a solvated Ti(IV) ion with high affinity for transferrin. As for Ga(III), selective transport of titanium ions via the transferrin route appears plausible. In vitro, titanocene dichloride is active in cisplatin-resistant cancer cells. It entered clinical trials in 1993, revealing nephrotoxicity as dose-limiting side effect. In Phase II studies as singleagent therapy no advantage over other treatment regimens was observed, and the trials were thus abandoned.
Titanocene dichloride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| WUXI HONOR SHINE CHEMICAL CO.,LTD | 0510-83593312 +8613665125081 | sales@honorshinechem.com | China | 61 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7786 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Fluoropharm Co., Ltd. | +86-0571-85586753; +8613336034509 | sales@fluoropharm.com | China | 1290 | 60 |
| Tianjin Zhongxin Chemtech Co., Ltd. | +86-022-66880623 +8618622897568 | sales@tjzxchem.com | China | 559 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Accela ChemBio Inc. | (+1)-858-699-3322 | info@accelachem.com | United States | 19965 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
Related articles
- Titanocene dichloride: Overview, Applications in the Synthesis of Hollow TiO2 Nanostructures and Safety
- Titanocene dichloride shows promise in cancer treatment and nanomaterial synthesis, despite clinical challenges and safety con....
- Feb 23,2024
- Applications of Titanocene dichloride
- Titanocene Dichloride is a useful reagent for a wide variety of synthetic transformations. It can be used with Grignard reagen....
- Mar 15,2023
- Preparation of Titanocene dichloride
- Titanocene dichloride is mainly used as a catalyst for olefin copolymerization and homopolymerization.
- Jun 17,2022
View Lastest Price from Titanocene dichloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-16 | titanocene dichloride
1271-10-8
|
US $0.00-0.00 / kg | 1kg | ≥99.0% | 60tons | 岳阳市金茂泰科技有限公司 | |
 |
2024-01-18 | bis-cyclopentadienyl titanium dichloride
1271-19-8
|
US $6.00-0.60 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2023-08-02 | Titanocene Dichloride
1271-19-8
|
US $0.00-0.00 / Kg/Drum | 20Kg/Drum | 99% | 50tons | WUXI HONOR SHINE CHEMICAL CO.,LTD |
-

- titanocene dichloride
1271-10-8
- US $0.00-0.00 / kg
- ≥99.0%
- 岳阳市金茂泰科技有限公司
-

- bis-cyclopentadienyl titanium dichloride
1271-19-8
- US $6.00-0.60 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- Titanocene Dichloride
1271-19-8
- US $0.00-0.00 / Kg/Drum
- 99%
- WUXI HONOR SHINE CHEMICAL CO.,LTD