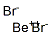beryllium dibromide
- CAS No.
- 7787-46-4
- Chemical Name:
- beryllium dibromide
- Synonyms
- Einecs 232-115-9;Beryllium bromide.;beryllium dibromide;Beryllium bromide (bebr2);beryllium(+2) cation dibromide
- CBNumber:
- CB3926651
- Molecular Formula:
- BeBr2
- Molecular Weight:
- 168.820182
- MDL Number:
- MOL File:
- 7787-46-4.mol
| Melting point | 506-509°; also reported as 488° |
|---|---|
| Boiling point | bp 520° |
| Density | 3.465 |
| solubility | very soluble in H2O; soluble in ethanol, pyr |
| form | orthorhombic crystals |
| color | orthorhombic crystals, crystalline; hygroscopic |
| Water Solubility | very soluble H2O [MER06] |
| FDA UNII | T00751H2J8 |
| EPA Substance Registry System | Beryllium bromide (BeBr2) (7787-46-4) |
beryllium dibromide Chemical Properties,Uses,Production
Chemical Properties
orthorhombic; very hygroscopic; -80 mesh, 99% purity; enthalpy of fusion 9.80 kJ/mol; made by reaction of Be and Br2 at 500°C–700°C [KIR78] [CER91] [MER06] [CRC10]
Uses
Beryllium fluoride is used in biochemistry, particularly protein crystallography, since it binds in some of the same ways as phosphate does in human tissues. ADP and beryllium fluoride together tend to bind to ATP sites and inhibit protein action, making
Preparation
Beryllium bromide has the formula BeBr2. It is very
hygroscopic and is soluble in water. It has the molecular
weight of 168.821 g/mol. The structure has not been
documented. Beryllium bromide can be prepared by
reacting Be metal with elemental bromine at temperatures
of 500°C–700°C:
Be+Br2→BeBr2
Beryllium bromide is also formed when beryllium
oxide with HBr in aqueous solution or hydrogen
bromide in the gas phase:
BeO+2HBr→BeBr2+H2O
This bromide compound was first prepared by Wohler
(1828) by the action of bromine vapor on the metal
and also upon a mixture of carbon and beryllium oxide.Berthemot (1831) obtained it in solution by dissolving
the oxide in hydrobromic acid. Humpridge (1883) also
prepared it by acting on a mixture of the oxide and
carbon with dry bromine.
The anhydrous bromide is always obtained by sublimation
as colorless white crystals.
beryllium dibromide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9116 | 58 |
| Hu Bei Jiutian Bio-medical Technology CO.,Ltd | 027-88013699 17354350817 | Ryan@jiutian-bio.com | China | 7433 | 58 |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 17691182729 18161915376 | 1046@dideu.com | China | 10008 | 58 |