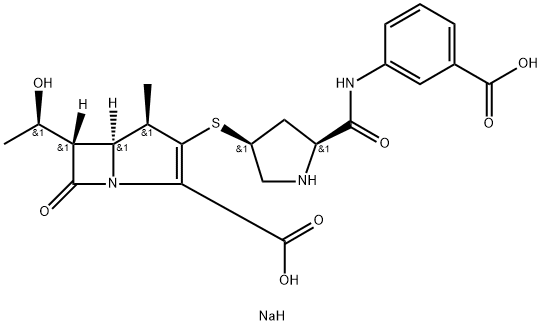
Ertapenem Disodium
- CAS No.
- 153832-38-3
- Chemical Name:
- Ertapenem Disodium
- Synonyms
- ERTAPENEM NA;Ertapenem disodium;Ertapenam disodium;Ertapenem Disodium 90%;ErtapeneM SodiuM(crude);Ertapenem (sodium salt);Ertapenem Disodium (85%);Ertapenem Disodium USP/EP/BP;1-Azabicyclo(3.2.0)hept-2-ene-2-carboxylic acid;sodium 3-((2S,4S)-4-(((4R,5S,6S)-2-carboxy-6-((R)-1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-en-3-yl)thio)pyrrolidine-2-carboxamido)benzoate
- CBNumber:
- CB41261369
- Molecular Formula:
- C22H26N3NaO7S
- Molecular Weight:
- 499.51
- MDL Number:
- MFCD00922233
- MOL File:
- 153832-38-3.mol
- MSDS File:
- SDS
| Melting point | 230-234°C (dec.) |
|---|---|
| storage temp. | Sealed in dry,2-8°C |
| solubility | Methanol (Slightly), Water (Slightly) |
| form | Solid |
| color | White to Off-White |
| Stability | Unstable at room temperature! |
| FDA UNII | P5HEA33D9R |
| NCI Drug Dictionary | ertapenem sodium |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319 | |||||||||
| Precautionary statements | P264-P280-P302+P352-P305+P351+P338-P332+P313-P337+P313-P362 | |||||||||
| NFPA 704 |
|
Ertapenem Disodium price More Price(16)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 20523 | Ertapenem (sodium salt) ≥98% | 153832-38-3 | 1mg | $32 | 2024-03-01 | Buy |
| Cayman Chemical | 20523 | Ertapenem (sodium salt) ≥98% | 153832-38-3 | 5mg | $109 | 2024-03-01 | Buy |
| Cayman Chemical | 20523 | Ertapenem (sodium salt) ≥98% | 153832-38-3 | 10mg | $184 | 2024-03-01 | Buy |
| Cayman Chemical | 20523 | Ertapenem (sodium salt) ≥98% | 153832-38-3 | 25mg | $381 | 2024-03-01 | Buy |
| TRC | E635000 | ErtapenemDisodium90% | 153832-38-3 | 100mg | $820 | 2021-12-16 | Buy |
Ertapenem Disodium Chemical Properties,Uses,Production
Description
Ertapenem sodium was introduced in the US as a once daily intravenous or intramuscular injection for the treatment of adult patients with moderate to severe bacterial infections. This new I-β-methyl carbapenem can be assembled from commercially available 4-nitrobenzyl protected β-methyl carbapenem enolphosphate and the appropriate thiol derivative. The last intermediate can be synthesized from a suitably N-protected 4- hydroxy proline derivative in a one pot operation involving bis activation of the carboxy and hydroxy groups, reaction with sodium sulfide yielding the corresponding thiolactone, aminolysis with 2-aminobenzoic acid and Kdeprotection. This bacterial cell wall synthesis inhibitor has broad spectrum antimicrobial activity including common Gram-positive and Gram-negative aerobic pathogens and restricted activity against nosocomial pathogens such as Pseudomonas aeruginosa, Acinetobacfer species, methicillin-resistant staphylococci and enterococci. Ertapenem is resistant to a broad and extended spectrum of β-lactamases (excluding metallo-beta-lactamase) and is also more resistant than imipenem to human renal dehydroxypeptidase-I-inactivation (DHP-I). In drug-resistant strains of P. aeruginosa, resistance to ertapenem and imipenem was common but almost all strains remain susceptible to at least one antipseudomal agent. In phase III studies, ertapenem showed efficacy in the treatment of obstetric and gyneacological infections, skin and soft tissues infections, community-acquired pneumonia, urinary tract infections and in intra abdominal infections. Ertapenem has improved pharmacokinetics over currently available carbapenems and cephalosporins with an extended serum half-life of 4h. The overall safety and tolerability profile of ertapenem was comparable to that seen with comparator antibacterials.
Chemical Properties
Pale Yellow Solid
Originator
Astra Zeneca (UK)
Uses
Group 1 carbapenem antibiotic. An antibacterial.
brand name
lnvanz
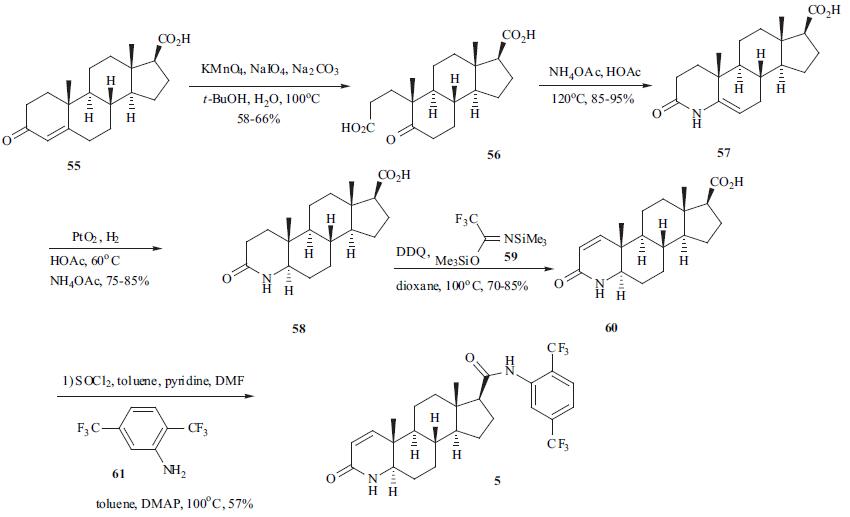
Synthesis
Following a conventional carbapenem synthetic strategy, ertapenem sodium (6) can be assembled from 4-nitrobenzylprotected |?-methyl carbapenemenolphosphate 71 and 2- aminocarbonylpyrrolidine-4-ylthio-containing side chain 70. Many efficient approaches to 71 have been reported in the literature , and this compound is now commercially available on a large scale. The synthesis of 70 is outlined in the scheme. Protection of the amino group in trans-4-hydroxy-L-proline (62) with diisopropyl phosphite followed by NaClO oxidation gave N-DIPP protected hydroxyl proline 63 in 80% yield. The carboxyl group in 63 was activated via reaction with diphenylphosphinic chloride (DPPC) in the presence of diisopropylethylamine (DIPEA). This intermediate 64 was directly reacted with methanesulfonyl chloride in the presence of pyridine to furnish mesylate 65. Mesylate 65 was then quenched with aqueous sodium sulfide yielding 66 instantaneously, which then slowly cyclized to 67. Aminolysis of 67 with m-aminobenzoic acid (68) and subsequent deprotection of the DIPP group with concentrated HCl provided 70 in 90-95% yield in a one-pot process. The coupling reaction between 70 and 71 followed by deprotection of PNB group was completed in one reaction vessel to furnish ertapenem sodium (6) (yield was not disclosed).
in vitro
in e.coli, ertapenem binds to penicillin binding proteins (pbps) 1a, 1b, 2, 3, 4 and 5, showing highest affinity for pbps 2 and 3 [1]. mic90s for most species of enterobacteriaceae were < 1 mg/l. mic90s for most bacteroides fragilis group isolates ranged from 1 to 4 mg/l, and mic90s were species specific for clostridium, ranging from 0.06 mg/l for clostridium perfringens to 4 mg/l for clostridiumclostridioforme [2].
in vivo
in healthy young men and women volunteers, the mean concentration of ertapenem in plasma ranged from ~145 to 175 μg/ml at the end of a 30-min infusion, from ~30 to 34 μg/ml at 6 h, and from ~9 to 11 μg/ml at 12 h. the mean plasma t1/2 ranged from 3.8 to 4.4 h. about 45% of the plasma clearance (clp) was via renal clearance [3].
References
[1]. shah p m, isaacs r d. ertapenem, the first of a new group of carbapenems[j]. journal of antimicrobial chemotherapy, 2003, 52(4): 538-542.
[2]. wexler h m. in vitro activity of ertapenem: review of recent studies[j]. journal of antimicrobial chemotherapy, 2004, 53(suppl 2): ii11-ii21.
[3]. majumdar a k, musson d g, birk k l, et al. pharmacokinetics of ertapenem in healthy young volunteers[j]. antimicrobial agents and chemotherapy, 2002, 46(11): 3506-3511.
Ertapenem Disodium Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-18612256290 | 1056@dideu.com | China | 3581 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9320 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29220 | 58 |
View Lastest Price from Ertapenem Disodium manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-10 | Ertapenem Disodium
153832-38-3
|
US $0.00-0.00 / mg | 10mg | 90%+ | 10g | Guangzhou PI PI BIOTECH INC | |
 |
2023-08-18 | Ertapenem Disodium
153832-38-3
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2021-11-09 | Ertapenem Disodium
153832-38-3
|
US $10.00 / KG | 100KG | 99% | 100 mt | Hebei Guanlang Biotechnology Co., Ltd. |
-

- Ertapenem Disodium
153832-38-3
- US $0.00-0.00 / mg
- 90%+
- Guangzhou PI PI BIOTECH INC
-

- Ertapenem Disodium
153832-38-3
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Ertapenem Disodium
153832-38-3
- US $10.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
153832-38-3(Ertapenem Disodium)Related Search:
1of4