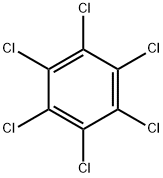
HEXACHLOROBENZENE
- CAS No.
- 118-74-1
- Chemical Name:
- HEXACHLOROBENZENE
- Synonyms
- Hexachlorocyclohexane;HCB;Hexachlorbenzene;PERCHLOROBENZENE;Hexachlorbenzol;BENZENE HEXACHLORIDE;Benzene, hexachloro-;granox;hexacb;Amatin
- CBNumber:
- CB4149734
- Molecular Formula:
- C6Cl6
- Molecular Weight:
- 284.78
- MDL Number:
- MFCD00000540
- MOL File:
- 118-74-1.mol
- MSDS File:
- SDS
| Melting point | 227-229 °C(lit.) |
|---|---|
| Boiling point | 323-326 °C(lit.) |
| Density | 1.5691 |
| vapor pressure | 1.45 x l0-3 Pa (20 °C) |
| refractive index | 1.5691 (estimate) |
| Flash point | 11 °C |
| storage temp. | APPROX 4°C |
| solubility | Chloroform, Hexanes (Slightly) |
| Water Solubility | Practically insoluble in water |
| Merck | 13,4696 |
| BRN | 1912585 |
| Henry's Law Constant | 6.11 at 25 °C (continuous flow sparger, Sproule et al., 1991) |
| Exposure limits | No exposure limit has been set for this com pound. Carcinogenicity: Animal Sufficient Evidence, Human Limited Evidence (IARC). |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | CKAPSXZOOQJIBF-UHFFFAOYSA-N |
| EPA Primary Drinking Water Standard | MCL:0.001,MCLG:zero |
| FDA 21 CFR | 165.126 |
| CAS DataBase Reference | 118-74-1(CAS DataBase Reference) |
| EWG's Food Scores | 10 |
| FDA UNII | 4Z87H0LKUY |
| IARC | 2B (Vol. Sup 7, 79) 2001 |
| Proposition 65 List | Hexachlorobenzene |
| Proposition 65 List | Hexachlorocyclohexane (Technical Grade) |
| Pesticides Freedom of Information Act (FOIA) | Hexachlorobenzene |
| EPA Substance Registry System | Hexachlorobenzene (118-74-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H350-H372-H410 |
| Precautionary statements | P202-P260-P264-P270-P273-P308+P313 |
| Hazard Codes | T,N,Xn,F,Xi |
| Risk Statements | 45-48/25-50/53-67-65-62-51/53-48/20-38-11-39/23/24/25-23/24/25-66-36/38-36 |
| Safety Statements | 53-45-60-61-62-36/37-33-29-16-9-26 |
| RIDADR | UN 2729 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | DA2975000 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| HS Code | 2903920000 |
| Toxicity | LC50 for ?ve freshwater species 0.05-0.2 mg/L (Hartley and Kidd, 1987); acute oral LD50 for rats 10,000 mg/kg (RTECS, 1985). |
HEXACHLOROBENZENE price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 45522 | Hexachlorobenzene PESTANAL | 118-74-1 | 250mg | $45 | 2024-03-01 | Buy |
| Sigma-Aldrich | 40008 | Hexachlorobenzene solution certified reference material, 1000?μg/mL in acetone | 118-74-1 | 1mL | $40.8 | 2024-03-01 | Buy |
| TRC | H290815 | Hexachlorobenzene | 118-74-1 | 100g | $1070 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | HCH0026187 | HEXACHLOROBENZENE 95.00% | 118-74-1 | 25G | $1209.36 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | HCH0026187 | HEXACHLOROBENZENE 95.00% | 118-74-1 | 100G | $2490.45 | 2021-12-16 | Buy |
HEXACHLOROBENZENE Chemical Properties,Uses,Production
Description
Hexachlorobenzene is a white crystalline solid. This compound does not occur naturally. It is formed as a by-product during the manufacture of chemicals used as solvents (substances used to dissolve other substances), other chlorine-containing compounds, and pesticides. Small amounts of hexachlorobenzene can also be produced during combustion processes such as burning of city wastes. It may also be produced as a by-product in waste streams of chlor-alkali and wood-preserving plants. Hexachlorobenzene was widely used as a pesticide until 1965. It was also used to make fireworks, ammunition, and synthetic rubber.
Chemical Properties
Hexachlorobenzene is a solid, crystallizing in nee dles.
Chemical Properties
white powder
Uses
Hexachlorobenzene is used as a fungicideand as an intermediate in organic synthesis.
Uses
Seed fungicide
Uses
No commercial uses of hexachlorobenzene as an end product in the United States were identified (ATSDR 2002). Previously, it was used as a seed-treatment fungicide for onions, sorghum, wheat, and other grains (IARC 1979). All registered pesticide uses in the United States were voluntarily cancelled in 1984 (ATSDR 2002). Hexachlorobenzene was also used as a chemical intermediate in dye manufacturing, in the synthesis of other organic chemicals, and in the production of pyrotechnic compositions for the military. It was used as a raw material for synthetic rubber, as a plasticizer for polyvinyl chloride, as a porosity controller in the manufacture of electrodes, and as a wood preservative (IARC 1979, ATSDR 2002).
Uses
In organic syntheses. Formerly as agricultural fungicide.
Definition
hexachlorobenzene: A colourlesscrystalline compound, C6Cl6; m.p.227°C. It is made by the chlorinationof benzene with an iron(III) chloridecatalyst or by treating hexachlorocyclohexanewith chlorine in hexachloroethane.It is used to preservewood and dress seeds, and in themanufacture of hexafluorobenzene.
Definition
ChEBI: A member of the class of chlorobenzenes that is benzene in which all of the hydrogens are replaced by chlorines. An agricultural fungicide introduced in the mid-1940s and formerly used as a seed treatment, its use has been banned since 1984 under the Stock olm Convention on Persistent Organic Pollutants.
Synthesis Reference(s)
Journal of the American Chemical Society, 69, p. 3146, 1947 DOI: 10.1021/ja01204a507
General Description
A white crystalline substance. Insoluble in water and denser than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion. Used to make other chemicals.
Air & Water Reactions
HEXACHLOROBENZENE is sensitive to moisture. Insoluble in water.
Reactivity Profile
HEXACHLOROBENZENE reacts violently with dimethylformamide. .
Hazard
Possible carcinogen. Toxic by ingestion. Combustible.
Health Hazard
The acute oral and inhalation toxicity ofhexachlorobenzene is low in test animals.Repeated ingestion of this compound mayproduce porphyria hepatica (increased for mation and excretion of porphyrin) causedby disturbances in liver metabolism. The oralLD50 value in rabbits is 2600 mg/kg; theinhalation LC50 value from a single exposureis 1800 mg/m3 (NIOSH 1986). The occupa tional health hazard from inhalation shouldbe very low because of its very low vaporpressure (0.00001 torr).
Hexachlorobenzene causes cancer in ani mals. Oral administration of this compoundfor 18 weeks to 2 years caused tumors inthe liver, kidney, thyroid, and blood in rats,mice, and hamsters. It is a suspected humancarcinogen, evidence of which occurs to alimited extent.
Health Hazard
Harmful by dust inhalation or if swallowed. Irritating to eyes, skin and mucous membranes. Prolonged periods of ingestion may cause cutaneous porphyria.
Fire Hazard
Noncombustible solid; very low reactiv ity. Reaction with dimethyl formamide is reported to be violent at temperatures above 65°C (149°F) (NFPA 1997).
Potential Exposure
Hexachlorobenzene was used as a fun gicide; an additive for pyrotechnic compositions; and as wood preservative. It was used widely as a pesticide to pro tect seeds of onions and sorghum, wheat, and other grains against fungus until 1965. This material was used to make fireworks; ammunition for military uses; synthetic rubber; as a porosity controller in the manufacture of electrodes; as an intermediate in dye manufacture; in organic synthesis. It is formed as a by-product of making other chemicals; in the waste streams of chloralkali and wood-preserving plants; and when burning municipal waste. Currently, there are no commercial uses of hexachlorobenzene in the United States.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, includ-ing resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medi-cal attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Note to physician: For ingestions of less than 10 mg/kg bodyweight occurring less than an hour before treatment, induceemesis. For ingestions of more than 10 mg/kg body weightoccurring less than an hour before treatment, use gastriclavage. For ingestion occurring more than an hour beforetreatment, use activated charcoal. There is no specific anti-dote, and supervision for at least 72 h is recommended.
Carcinogenicity
Hexachlorobenzene is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Source
Hexachlorobenzene may enter the environment from incomplete combustion of chlorinated compounds including mirex, kepone, chlorobenzenes, pentachlorophenol, PVC, polychlorinated biphenyls, and chlorinated solvents (Ahling et al., 1978; Dellinger et al., 1991). In addition, hexachlorobenzene may enter the environment as a reaction by-product in the production of carbon tetrachloride, dichloroethylene, hexachlorobutadiene, trichloroethylene, tetrachloroethylene, pentachloronitrobenzene, and vinyl chloride monomer (quoted, Verschueren, 1983).
Environmental Fate
Biological. Reductive monodechlorination occurred in an anaerobic sewage sludge yielding principally 1,3,5-trichlorobenzene. Other compounds identified included pentachlorobenzene, 1,2,3,5-tetrachlorobenzene and dichlorobenzenes (Fathepure et al., 1988). In activated sludge, only 1.5% of the applied hexachlorobenzene mineralized to carbon dioxide after 5 days (Freitag et al., 1985). In a 5-day experiment, 14C-labeled hexachlorobenzene applied to soil-water suspensions under aerobic and anaerobic conditions gave 14CO2 yields of 0.4 and 0.2%, respectively (Scheunert et al., 1987).
When hexachlorobenzene was statically incubated in the dark at 25°C with yeast extract and settled domestic wastewater inoculum, no signi?cant biodegradation was observed. At a concentration of 5 mg/L, percent losses after 7, 14, 21 and 28-day incubation
Groundwater. According to the U.S. EPA (1986) hexachlorobenzene has a high potential to leach to groundwater.
Photolytic. Solid hexachlorobenzene exposed to arti?cial sunlight for 5 months photolyzed at a very slow rate with no decomposition products identified (Plimmer and Klingebiel, 1976). The sunlight irradiation of hexachlorobenzene (20 g) in a 100 mL borosilicate glass-stoppered Erlenmeyer ?ask for 56 days yielded 64 ppm pentachlorobiphenyl (Uyeta et al., 1976). A carbon dioxide yield <0.1% was observed when hexachlorobenzene adsorbed on silica gel was irradiated with light (λ >290 nm) for 17 hours (Freitag et al., 1985).
Irradiation (λ ≥285 nm) of hexachlorobenzene (1.1–1.2 mM/L) in an acetonitrile-water mixture containing acetone (concentration = 0.553 mM/L) as a sensitizer gave the following products (% yield): pentachlorobenzene (71.0), 1,2,3,4-tetrachlorobenzene (0.6)
Metabolic pathway
With the incubation of rat liver microsomes, hexachlorobenzene is metabolized to give pentachlorophenol and tetrachlorohydroquinone, and, in addition, a considerable amount of covalent binding to protein is detected (250 pM pentachlorophenol, 17 pM tetrachlorohydroquinone, and 11 pM tetrachlorobenzoquinone covalent binding in an incubation containing 50 μM hexachlorobenzene).
Metabolism
Sensitized photolysis of HCB at wavelengths greater
than 285 nm in acetonitrile/water containing acetone gave
dechlorinated products: pentachlorobenzene (78) (71%),
1,2,3,4-tetrachlorobenzene (79) (0.6%), 1,2,3,5-tetrachlorobenzene
(80) (2.2%), and 1,2,4,5- tetrachlorobenzene
(81) (3.7%). Without acetone, products included
pentachlorobenzene (78) (76.8%), 1,2,3,5-tetrachlorobenzene
(80) (1.2%), 1,2,4,5- tetrachlorobenzene (81) (1.7%),
and 1,2,4-trichlorobenzene (82) (0.2%) (105).
Irradiation of hexachlorobenzene in methanol solution
at wavelengths greater than 260 nm gave a
mixture of reductively dechlorinated products (pentachlorobenzene
and a tetrachlorobenzene, probably 80)
and pentachlorobenzyl alcohol 83, and also a tetrachlorodi(
hydroxymethyl)benzene (106). A similar product
mixture was obtained by exposing a methanolic solution of
hexachlorobenzene inmethanol to sunlight outdoors. After
15 days, only 30% of hexachlorobenzene was recovered.
Photolysis rates were enhanced by the addition of sensitizers
(diphenylamine, tryptophane, and naturally occurring
organic substances), but no products were identified.
In an anaerobic sewage sludge, hexachlorobenzene was
reductively dechlorinated and the principal product was
1,3,5-trichlorobenzene (84). Pentachlorobenzene, 1,2,3,5-
tetrachlorobenzene, and dichlorobenzenes were also identified
(107). In activated sludge, 1.5% of hexachlorobenzene
was mineralized as carbon dioxide after 5 days.
Solubility in organics
In millimole fraction at 25 °C: 2.62 in n-hexane, 3.14 in n-heptane, 3.71 in n-octane, 4.10 in nnonane, 4.60 in n-decane, 6.81 in n-hexadecane, 2.95 in cyclohexane, 3.87 in methylcyclohexane, 2.52 in 2,2,4-trimethylpentane, 4.71 in tert-butylcyclohexane, 4.40 in dibutyl ether, 3.20 in methyl tert-butyl ether, 5.92 in tetrahydrofuran, 3.97 in 1,4-dioxane, 0.0902 in methanol, 0.236 in ethanol, 0.398 in 1-propanol, 0.298 in 2-propanol, 0.667 in 1-butanol, 0.521 in 2- butanol, 0.533 in 2-methyl-1-propanol, 0.517 in 2-methyl-2-propanol, 1.03 in 1-pentanol, 0.860 in 2-propanol, 0.770 in 3-methyl-1-butanol, 1.20 on 2-methyl-2-butanol, 1.44 in 1-hexanol, 1.40 in 2-methyl-1-pentanol, 1.43 in 4-methyl-2-pentanol, 1.90 in 1-heptanol, 2.38 in 1-octanol, 1.74 in 2-ethyl-1-hexanol, 3.80 in 1-decanol, 0.920 in cyclopentanol, 3.65 in butyl acetate, 2.11 in ethyl acetate, 1.48 in methyl acetate, 2.86 in 1,2-dichloroethane, 3.83 in 1-chlorobutane, 5.08 in 1-chlorohexane, 6.06 in 1-chlorooctane, 6.10 in chlorocyclohexane (De Fina et al., 2000)
Solubility in water
In millimole fraction at 25 °C: 2.62 in n-hexane, 3.14 in n-heptane, 3.71 in n-octane, 4.10 in nnonane, 4.60 in n-decane, 6.81 in n-hexadecane, 2.95 in cyclohexane, 3.87 in methylcyclohexane, 2.52 in 2,2,4-trimethylpentane, 4.71 in tert-butylcyclohexane, 4.40 in dibutyl ether, 3.20 in methyl tert-butyl ether, 5.92 in tetrahydrofuran, 3.97 in 1,4-dioxane, 0.0902 in methanol, 0.236 in ethanol, 0.398 in 1-propanol, 0.298 in 2-propanol, 0.667 in 1-butanol, 0.521 in 2- butanol, 0.533 in 2-methyl-1-propanol, 0.517 in 2-methyl-2-propanol, 1.03 in 1-pentanol, 0.860 in 2-propanol, 0.770 in 3-methyl-1-butanol, 1.20 on 2-methyl-2-butanol, 1.44 in 1-hexanol, 1.40 in 2-methyl-1-pentanol, 1.43 in 4-methyl-2-pentanol, 1.90 in 1-heptanol, 2.38 in 1-octanol, 1.74 in 2-ethyl-1-hexanol, 3.80 in 1-decanol, 0.920 in cyclopentanol, 3.65 in butyl acetate, 2.11 in ethyl acetate, 1.48 in methyl acetate, 2.86 in 1,2-dichloroethane, 3.83 in 1-chlorobutane, 5.08 in 1-chlorohexane, 6.06 in 1-chlorooctane, 6.10 in chlorocyclohexane (De Fina et al., 2000)
storage
Color Code- Blue: Health Hazard/Poison: Storeinia securepoison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, well-ventilated area away from oxidizers, dimethyl formamide,and heat. Where possible, automatically pump liquid fromdrums or other storage containers to process containers. Aregulated, marked area should be established where thischemical is handled, used, or stored in compliance withOSHA Standard 1910.1045.
Shipping
UN2729 Hexachlorobenzene, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Crystallise hexachlorobenzene repeatedly from *benzene. Dry it under vacuum over P2O5. [Beilstein 5 H 205, 5 IV 670.]
Degradation
Hexachlorobenzene is very stable and is unreactive toward acids and
bases.
Photolysis is very slow and in artificial sunlight, solid HCB photodecomposed
after 5 months. In sunlight, 20 g of HCB contained in a borosilicate flask gave a concentration of 64 mg kg-1 of pentachlorobiphenyl
after 56 days (Uyeta et al., 1976).
Sensitised photolysis of HCB in an acetonitrile/water mixture containing
acetone at wavelengths greater than 285 nm gave the following products:
pentachlorobenzene (2) (71%), 1,2,3,4-tetrachlorobenzene (3)
(0.6%), 1,2,3,5-tetrachlorobenzene (4) (2.2%) and 1,2,4,5-tetrachlorobenzene
(5) (3.7%). In the absence of acetone, products identified included
2 (76.8%), 4 (1.2%), 5 (1.7%) and 1,2,4-trichlorobenzene (6) (0.2%)
(Choudhry and Hutzinger, 1984) (see Scheme 1).
Toxicity evaluation
There are no reports of avian casualties, although raptors found dead in The Netherlands had substantial levels of HCB in their livers along with cyclodiene and DDE residues (33). The same authors reported porphyria in quail following a 3-month dosing period with 20-ppm HCB. Product registrations in Canada at the time allowed up to 1000 ppm on various cereal seeds. In the early 1970s, levels in the range of 3–4 ppm (fresh weight basis) were seen in eggs of fish-eating birds of the Great Lakes and likely contributed to the high levels of embryonic mortality seen (34). However, because HCB is also an intermediate in the manufacture of several chemicals, industrial pollution rather than use of the chemical on farm fields could have been the source of the contamination.
Incompatibilities
Reacts violently with oxidizers; dimethyl formamide above 65 ℃.
Waste Disposal
Incineration is most effective @ 1300 ℃ and 0.25 seconds. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal.
HEXACHLOROBENZENE Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9360 | 58 |
| BeiJing Hwrk Chemicals Limted | 0757-86329057 18501085097 | sales3.gd@hwrkchemical.com | China | 7583 | 55 |
View Lastest Price from HEXACHLOROBENZENE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2020-01-09 | HEXACHLOROBENZENE
118-74-1
|
US $9.80 / KG | 1g | ≥99% | 100kg | Career Henan Chemical Co |
-

- HEXACHLOROBENZENE
118-74-1
- US $9.80 / KG
- ≥99%
- Career Henan Chemical Co