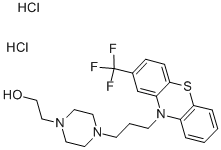
FLUPHENAZINE HYDROCHLORIDE
- CAS No.
- 146-56-5
- Chemical Name:
- FLUPHENAZINE HYDROCHLORIDE
- Synonyms
- FLUPHENAZINE HCL;FLUPHENAZINE DIHYDROCHLORIDE;Prolinate;prolixin;anatensol;omca;a4077;liogen;moditen;mirenil
- CBNumber:
- CB4154808
- Molecular Formula:
- C22H28Cl2F3N3OS
- Molecular Weight:
- 510.44
- MDL Number:
- MFCD00055212
- MOL File:
- 146-56-5.mol
- MSDS File:
- SDS
| Melting point | 200-2020C |
|---|---|
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO and methanol. |
| color | White to Light yellow |
| Sensitive | Hygroscopic |
| Stability | Hygroscopic |
| FDA UNII | ZOU145W1XL |
| NCI Dictionary of Cancer Terms | fluphenazine hydrochloride |
| NCI Drug Dictionary | fluphenazine hydrochloride |
| EPA Substance Registry System | Fluphenazine dihydrochloride (146-56-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H360 | |||||||||
| Precautionary statements | P201-P280-P301+P330+P331+P310-P308+P313 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 36/37/38-20/21/22 | |||||||||
| Safety Statements | 26-36/37/39-36 | |||||||||
| RIDADR | UN2811 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TL9800000 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 2934302300 | |||||||||
| Toxicity | guinea pig,LD50,intraperitoneal,299mg/kg (299mg/kg),Pharmazie. Vol. 38, Pg. 749, 1983. | |||||||||
| NFPA 704 |
|
FLUPHENAZINE HYDROCHLORIDE price More Price(33)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | F-903 | Fluphenazine dihydrochloride solution 1.0?mg/mLinmethanol(asfreebase),ampuleof1?mL,certifiedreferencemateri | 146-56-5 | 1mL | $116 | 2024-03-01 | Buy |
| Sigma-Aldrich | BP167 | Fluphenazine hydrochloride British Pharmacopoeia (BP) Reference Standard | 146-56-5 | 100MG | $229 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1282004 | Fluphenazine hydrochloride United States Pharmacopeia (USP) Reference Standard | 146-56-5 | 125mg | $358 | 2024-03-01 | Buy |
| Sigma-Aldrich | F4765 | Fluphenazine dihydrochloride | 146-56-5 | 1g | $85.9 | 2023-06-20 | Buy |
| TCI Chemical | F1240 | Fluphenazine Dihydrochloride | 146-56-5 | 1G | $42 | 2024-03-01 | Buy |
FLUPHENAZINE HYDROCHLORIDE Chemical Properties,Uses,Production
Chemical Properties
Off-White Solid
Originator
Prolixin, Squibb ,US ,1959
Uses
antiandrogen, antineoplastic, Nuclear Hormone receptor antagonist
Uses
Antipsychotic
Definition
ChEBI: Fluphenazine hydrochloride is a member of phenothiazines. It has a role as an anticoronaviral agent.
Manufacturing Process
A suspension of 69.0 grams of 2-trifluoromethylphenothiazine in 1 liter of toluene with 10.9 grams of sodium amide is heated at reflux with high speed stirring for 15 minutes. A solution of 54.1 grams of 1-formyl-4-(3'chloropropyl)-piperazine, [prepared by formylating 1-(3'-hydroxypropyl)piperazine by refluxing in an excess of methyl formate, purifying the 1-formyl4-(3'-hydroxypropyl)-piperazine by vacuum distillation, reacting this compound with an excess of thionyl chloride at reflux and isolating the desired 1-formyl-4(3'-chloropropyl)-piperazine by neutralization with sodium carbonate solution followed by distillation] in 200 ml of toluene is added. The reflux period is continued for 4 hours. The cooled reaction mixture is treated with 200 ml of water. The organic layer is extracted twice with dilute hydrochloric acid. The acid extracts are made basic with ammonia and extracted with benzene. The volatiles are taken off in vacuo at the steam bath to leave a dark brown oil which is 10-[3'-(N-formylpiperazinyl)-propyl]-2trifluoromethylphenothiazine. It can be distilled at 260°C at 10 microns, or used directly without distillation if desired.
A solution of 103.5 grams of 10-[3'-(N-formylpiperazinyl)-propyl]-2trifluoromethylphenothiazine in 400 ml of ethanol and 218 ml of water containing 26 ml of 40% sodium hydroxide solution is heated at reflux for 2 hours. The alcohol is taken off in vacuo on the steam bath. The residue is swirled with benzene and water. The dried benzene layer is evaporated in vacuo. The residue is vacuum distilled to give a viscous, yellow oil, 10(3'piperazinylpropyl)-2-trifluoromethylphenothiazine, distilling at 210° to235°C at 0.5 to 0.6 mm.
A suspension of 14.0 grams of 10-(3'-piperazinylpropyl)-2trifluoromethylphenothiazine, 6.4 grams of β-bromoethyl acetate and 2.6 grams of potassium carbonate in 100 ml of toluene is stirred at reflux for 16 hours. Water (50 ml) is added to the cooled mixture. The organic layer is extracted into dilute hydrochloric acid. After neutralizing the extracts and taking the separated base up in benzene, a viscous, yellow residue is obtained by evaporating the organic solvent in vacuo. This oil is chromatographed on alumina. The purified fraction of 7.7 grams of 10-[3'-(Nacetoxyethylpiperazinyl)-propyl] -2-trifluoromethylphenothiazine is taken up in ethyl acetate and mixed with 25 ml of alcoholic hydrogen chloride. Concentration in vacuo separates white crystals of the dihydrochloride salt, MP 225° to 227°C.
A solution of 1.0 gram of 10-[3'-(N-acetoxyethylpiperazinyl)-propyl]-2trifluoromethylphenothiazine in 25 ml of 1 N hydrochloric acid is heated at reflux briefly. Neutralization with dilute sodium carbonate solution and extraction with benzene gives the oily base, 10-[3'-(N-βhydroxyethylpiperazinyl)-propyl]-2-trifluoromethylphenothiazine. The base is reacted with an excess of an alcoholic hydrogen chloride solution. Trituration with ether separates crystals of the dihydrochloride salt, MP 224° to 226°C, (from US Patent 3,058,979).
Therapeutic Function
Tranquilizer
General Description
The member ofthe piperazine subgroup with a trifluoromethyl group at the2-position of the phenothiazine system and the most potentantipsychotic phenothiazine on a milligram basis is fluphenazinehydrochloride, 4-[3-[2-(trifluoromethyl)phenazin-10-yl] propyl]-1-piperazineethanol dihydrochloride, 10[3-[4-(2-hydroxyethyl)piperazinyl]propyl]-2-trifluoromethylphenothiazine dihydrochloride (Permitil, Prolixin). It is alsoavailable as two lipid-soluble esters for depot intramuscularinjection, the enanthate (heptanoic acid ester) and the decanoateester. These long-acting preparations have use intreating psychotic patients who do not take their medicationor are subject to frequent relapse.
Biological Activity
ec50: 1.24 μmfluphenazine is a dopamine d1 and d2 receptor inhibitor.dopamine d1 and d2 receptor immunohistochemistry has been used to study the structure of the adult rat arcuate-median eminence complex, particularly in relation to the tubero-infundibular dopamine neurons.
Biochem/physiol Actions
D1/D2 dopamine receptor antagonist; phenothiazine antipsychotic; H1 histamine receptor antagonist.
in vitro
previous study showed that both phenothiazines of fluphenazine and perphenazine induced concentration-dependent loss in cell viability with ec50s to be 1.24 and 2.76 μm for fluphenazine and perphenazine, respectively. moreover, fluphenazine at 1.0 μm and perphenazine at 1.0 and 3.0 μm could inhibit melanogenesis and decrease the content of microphthalmia-associated transcription factor. in addition, both fluphenazine and perphenazine at higher concentrations caused depletion of melanocytes antioxidant status, indicating oxidative stress induction [1].
in vivo
systemic fluphenazine could effectively attenuate mechanical allodynia in rat neuropathic pain models at 0.03-0.3 mg/kg doses, which approximated those used in rodent models of psychosis. for antiallodynic effect, fluphenazine was able to effectively suppress the ectopic discharges in injured afferent fibers without affecting the propagation of action potentials in an ex-vivo drg-nerve preparation from cci rats [2].
References
[1] otreba m et al. fluphenazine and perphenazine impact on melanogenesis and antioxidant enzymes activity in normal human melanocytes. acta poloniae pharmaceutica-drug research, july-august 2016, 73(4):903-911.
[2] dong xw,jia y,lu sx,zhou x,cohen-williams m,hodgson r,li h,priestley t. the antipsychotic drug, fluphenazine, effectively reverses mechanical allodynia in rat models of neuropathic pain. psychopharmacology (berl). 2008 jan;195(4):559-68.
FLUPHENAZINE HYDROCHLORIDE Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10326 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| HONG KONG IPURE BIOLOGY CO.,LIMITED | 86 18062405514 18062405514 | ada@ipurechemical.com | CHINA | 3465 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6313 | 58 |
| Xi'an MC Biotech, Co., Ltd. | 029-89275612 +8618991951683 | mcbio_sales@163.com | China | 2255 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29271 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
View Lastest Price from FLUPHENAZINE HYDROCHLORIDE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-06-07 | Fluphenazine DiHCl
146-56-5
|
US $150.00 / kg | 1kg | 99.9% | 10000MT | Henan Bao Enluo International TradeCo.,LTD | |
 |
2021-07-24 | FLUPHENAZINE HYDROCHLORIDE USP/EP/BP
146-56-5
|
US $1.10 / g | 1g | 99.9% | 100 Tons min | Dideu Industries Group Limited | |
 |
2020-01-18 | FLUPHENAZINE HYDROCHLORIDE
146-56-5
|
US $3.00 / KG | 1KG | 98% | 1kg , 5kg, 50kg | Career Henan Chemical Co |
-

- Fluphenazine DiHCl
146-56-5
- US $150.00 / kg
- 99.9%
- Henan Bao Enluo International TradeCo.,LTD
-

- FLUPHENAZINE HYDROCHLORIDE USP/EP/BP
146-56-5
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited
-

- FLUPHENAZINE HYDROCHLORIDE
146-56-5
- US $3.00 / KG
- 98%
- Career Henan Chemical Co
146-56-5(FLUPHENAZINE HYDROCHLORIDE)Related Search:
1of4