ETHANE
- CAS No.
- 74-84-0
- Chemical Name:
- ETHANE
- Synonyms
- Dimethyl;C2H6;R170;Ethane,high purity;R-170;ETHANE;Bimethyl;ethylhydride;ETHANE, 99+%;ETHANE 99.9%
- CBNumber:
- CB4223139
- Molecular Formula:
- C2H6
Lewis structure
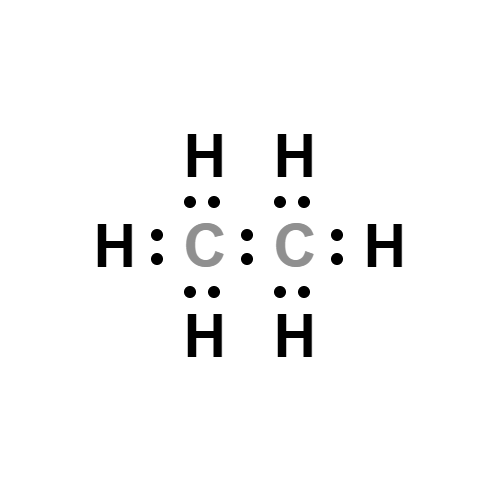
- Molecular Weight:
- 30.07
- MDL Number:
- MFCD00009023
- MOL File:
- 74-84-0.mol
| Melting point | −172 °C(lit.) |
|---|---|
| Boiling point | −88 °C(lit.) |
| Density | 0.362 g/mL at 20 °C(lit.) |
| vapor density | 1.05 (vs air) |
| vapor pressure | 37.95 atm ( 21.1 °C) |
| refractive index | 1.0047 |
| Flash point | −211 °F |
| form | gas |
| pka | 48(at 25℃) |
| Odor | odorless or mild gasoline-like odor |
| explosive limit | 13% |
| Water Solubility | 60.4mg/L(25 ºC) |
| Merck | 13,3758 |
| BRN | 1730716 |
| Stability | Stable. Highly flammable. Readily forms explosive mixtures with air. Incompatible with strong oxidizing agents. |
| LogP | 1.810 |
| CAS DataBase Reference | 74-84-0(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | L99N5N533T |
| EPA Substance Registry System | Ethane (74-84-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS04 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H220-H280 | |||||||||
| Precautionary statements | P410+P403 | |||||||||
| Hazard Codes | F+,F | |||||||||
| Risk Statements | 12 | |||||||||
| Safety Statements | 9-16-33 | |||||||||
| RIDADR | UN 1035 2.1 | |||||||||
| WGK Germany | - | |||||||||
| RTECS | KH3800000 | |||||||||
| F | 4.5-31 | |||||||||
| Autoignition Temperature | 881 °F | |||||||||
| Hazard Note | Flammable | |||||||||
| DOT Classification | 2.1 (Flammable gas) | |||||||||
| HazardClass | 2.1 | |||||||||
| HS Code | 2901100000 | |||||||||
| NFPA 704 |
|
ETHANE price More Price(14)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 539775 | Ethane 99.99% | 74-84-0 | 110g | $724 | 2024-03-01 | Buy |
| Sigma-Aldrich | 295302 | Ethane ≥99% | 74-84-0 | 110g | $604 | 2024-03-01 | Buy |
| Usbiological | 273194 | Dimethyl | 74-84-0 | 50mg | $296 | 2021-12-16 | Buy |
| Usbiological | 447223 | Dimethyl- | 74-84-0 | 25mg | $305 | 2021-12-16 | Buy |
| Usbiological | 447110 | Dimethyl | 74-84-0 | 1g | $312 | 2021-12-16 | Buy |
ETHANE Chemical Properties,Uses,Production
Uses
In the production of ethylene, vinyl chloride, and chlorinated hydrocarbons; as a component of bottled fuel gas.
Description
Ethane is a colorless, odorless, flammable gas that is relatively inactive chemically and is considered nontoxic. It is shipped as a liquefied compressed gas under its vapor pressure of 544 psig at 70°F (3750 kPa at 21.1℃).
Chemical Properties
ETHANE is colorless, odorless gas, practically insoluble in H2O, moderately soluble in alcohol. The compound burns when ignited in air with a pale faintly luminous flame; forms an explosive mixture with air over a moderate range. With excess air, products of combustion are CO2 and H2O. Ethane is among the chemically less reactive organic substances. However, ethane reacts with chlorine and bromine to form substitution compounds. Ethane occurs, usually in small amounts, in natural gas. The fuel value of ethane is high, 1,730 Btu per cubic foot. Ethane may be prepared by reaction of magnesium ethyl iodide in anhydrous ether (Grignard’s reagent) with H2O or alcohols. Ethyl iodide, ethyl bromide, or ethyl chloride, are preferably made by reaction with ethyl alcohol and the appropriate phosphorus halide. Important ethane derivatives, by successive oxidation, are ethyl alcohol, acetaldehyde, and acetic acid.
Chemical Properties
Ethane is a compressed, liquefied, colorless gas. Mild, gasoline-like odor. Odorless when pure.
History
Ethane was first synthesized in 1834 by Michael Faraday (1791–1867) through the electrolysis of acetate solutions, although Faraday believed the compound was methane. Twenty years later Adolph Wilhelm Hermann Kolbe (1818–1884) incorrectly identified ethane as the methyl radical in his research, and Edward Frankland (1825–1899) prepared ethane by treating ethyl iodine (C2H5I) with metals.
Uses
Ethane is the second major component in naturalgas. It is formed by petroleum cracking.It is used as a fuel gas, in the manufactureof chloro derivatives, and as a refrigerant.
Uses
It was first applied to the compound ether (CH3CH2OCH2CH3). Ether isa highly fl ammable compound that wasfirst prepared from the two-carbon alcohol ethanol(C2H5OH), and ethane is the two-carbon alkane. Ethane is the second most abundant componentof natural gas, with sources typically containing 1–5% by volume, but some sourcesmay contain up to 30% ethane.
Uses
In the manufacture of chlorinated derivatives; as refrigerant in some two-stage refrigeration systems where relatively low temperatures are produced; as fuel gas (so called "bottled gas" or "suburban propane" contains about 90% propane, 5% ethane, and 5% butane).
Production Methods
The synthesis of ethane takes place through a process called Kolbe synthesis. In this processacetic acid (CH3COOH) undergoes electrolysis to oxidize acetate ions at the anode of an electrochemicalcell to produce acetate radicals: CH3COO- → CH3COO?. Two acetate radicals thencombine to give ethane and carbon dioxide: CH3COO? + CH3COO? → C2H6 + 2CO2.
Definition
ethane: A colourless flammablegaseous hydrocarbon, C2H6; m.p.–183°C; b.p. –89°C. It is the secondmember of the alkane series of hydrocarbonsand occurs in natural gas.
Definition
A gaseous alkane obtained either from the gaseous fraction of crude oil or by the ‘cracking’ of heavier fractions. Ethane is the second member of the homologous series of alkanes.
General Description
A colorless odorless gas. ETHANE is easily ignited. The vapors are heavier than air. ETHANE can asphyxiate by the displacement of air. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. Contact with the liquid may cause frostbite.
Air & Water Reactions
Highly flammable.
Reactivity Profile
Saturated aliphatic hydrocarbons, such as ETHANE, may be incompatible with strong oxidizing agents like nitric acid. Charring of the hydrocarbon may occur followed by ignition of unreacted hydrocarbon and other nearby combustibles. In other settings, aliphatic saturated hydrocarbons are mostly unreactive. They are not affected by aqueous solutions of acids, alkalis, most oxidizing agents, and most reducing agents. Peroxidizable
Hazard
Severe fire risk if exposed to sparks or open flame. Flammable limits in air 3–12%. An asphyxiant gas.
Health Hazard
Like methane, ethane is a nonpoisonous gas.It is a simple asphyxiate. At high concentrationsit may exhibit narcotic effects.
Health Hazard
In high vapor concentrations, can act as simple asphyxiant. Liquid causes severe frostbite.
Fire Hazard
EXTREMELY FLAMMABLE. Will be easily ignited by heat, sparks or flames. Will form explosive mixtures with air. Vapors from liquefied gas are initially heavier than air and spread along ground. CAUTION: Hydrogen (UN1049), Deuterium (UN1957), Hydrogen, refrigerated liquid (UN1966) and METHANE (UN1971) are lighter than air and will rise. Hydrogen and Deuterium fires are difficult to detect since they burn with an invisible flame. Use an alternate method of detection (thermal camera, broom handle, etc.) Vapors may travel to source of ignition and flash back. Cylinders exposed to fire may vent and release flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.
Materials Uses
Ethane is noncorrosive and may be contained in installations constructed of any common metals designed to withstand the pressure involved.
Safety Profile
A simple asphyxiant. See ARGON for properties of simple asphyxiants. A very dangerous fire hazard when exposed to heat or flame; can react vigorously with oxidizing materials. Moderate explosion hazard when exposed to flame. To fight fire, stop flow of gas. Incompatible with chlorine, doxygenyl tetrafluoroborate, oxidizing materials, heat or flame. When heated to decomposition it emits acrid smoke and irritating fumes.
Potential Exposure
Ethane is used as a fuel, in making chemicals or as a freezing agent. The health effects caused by ethane exposure are much less serious than the fire and explosion risk posed by this chemical
Physiological effects
Inhalation of ethane in concentrations in air up to 5 percent produces no definite symptoms, but inhalation of higher concentrations has an anesthetic effect. It can act as a simple asphyxiant by displacing the oxygen in the air. Contact between liquid ethane and skin can cause freezing of the tissue.
First aid
Remove the person from exposure. Begin rescuebreathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart actionhas stopped. Transfer promptly to a medical facility. Iffrostbite has occurred, seek medical attention immediately;do NOT rub the affected areas or flush them with water. Inorder to prevent further tissue damage, do NOT attempt toremove frozen clothing from frostbitten areas. If frostbitehas NOT occurred, immediately and thoroughly wash contaminated skin with soap and water.
Carcinogenicity
Syrian hamster embryo cells were exposed in vitro to ethane gas. After exposure, the cells were removed and assayed for viability and increased sensitivity to viral transformation. Ethane was determined to be inactive.
storage
All the precautions required for the safe handling
of any flammable compressed gas must be
observed with ethane. It is important that ignition
sources be kept away from containers, including
situations where leakage could cause
the gas to ignite by such sources as a spark from
a motor. AlI piping and equipment used with
ethane should be grounded.
Ethane should not be stored with cylinders
containing oxygen, chlorine, or other oxidizing
or combustible materials.
Shipping
UN1035 (compressed gas); UN1961 (refrigerated liquid): Ethane, Hazard Class: 2.1; Labels: 2.1-Flammable gas. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.
Purification Methods
Ethylene can be removed by passing the gas through a sintered-glass disc into fuming H2SO4 then slowly through a column of charcoal saturated with bromine. Bromine and HBr are removed by passage through firebrick coated with N,N-dimethyl-p-toluidine. The ethane is also passed over KOH pellets (to remove CO2) and dried with Mg(ClO4)2. Further purification is by several distillations of liquified ethane, using a condensing temperature of -195o. Yang and Gant [J Phys Chem 65 1861 1961] treated ethane by standing it for 24hours at room temperature in a steel bomb with activated charcoal treated with bromine. They then immersed the bomb in a Dry-ice/acetone bath and transferred the ethane to an activated charcoal trap cooled in liquid nitrogen. (The charcoal had previously been degassed by pumping for 24hours at 450o.) By allowing the trap to warm slowly, the ethane distils, and only the middle third fraction is kept. Removal of methane is achieved using Linde type 13X molecular sieves (previously degassed by pumping for 24hours at 450o) in a trap which, after cooling in Dry-ice/acetone, is saturated with ethane. After pumping for 10minutes, the ethane is recovered by warming the trap to 25o. (The final gas contains less than 10-4 mole % of either ethylene or methane). [Beilstein 1 IV 108.]
Incompatibilities
Flammable gas; forms explosive mixture with air. Strong oxidizers may cause fire and explosions. May accumulate static electrical charges, and may cause ignition of its vapors.
Waste Disposal
Return refillable compressed gas cylinders to supplier. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
GRADES AVAILABLE
Ethane is typically available for commercial and industrial purposes in a c.P. grade (minimum purity of99.0 mole percent) or a technical grade (minimum purity of 95.0 mole percent).
ETHANE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81138252 +86-18789408387 | 1057@dideu.com | China | 3834 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9604 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| Guangzhou Yuejia Gas Co., Ltd | 400-6377517 19876107228 | linfeng@yigas.cn | China | 39 | 58 |
| Central China Special Gas (CCSG) Co., Ltd | 0734-8755555 15674722888 | lyq@ccsg.cn | China | 281 | 58 |
| Spectrum Chemical Manufacturing Corp. | 021-021-021-67601398-809-809-809 15221380277 | marketing_china@spectrumchemical.com | China | 9664 | 60 |
| Sigma-Aldrich | 021-61415566 800-8193336 | orderCN@merckgroup.com | China | 51471 | 80 |
| Shanghai wechem chemical co., ltd | 18824865657 | joey.lin@wechem.cn | China | 506 | 58 |
| AoboRui (Tianjin) Co., Ltd. | 022-65675308 18310521067 | 23034325@qq.com | China | 100 | 55 |
Related articles
- C2H6 lewis structure: Hybridization and Molecular Geometry
- The Lewis structure of C2H6 (ethane) consists of two carbon atoms and six hydrogen atoms (H)? at a bond angle of 109.5 degrees....
- Dec 14,2023
74-84-0(ETHANE)Related Search:
1of4






