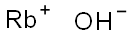Rubidium hydroxide
- CAS No.
- 1310-82-3
- Chemical Name:
- Rubidium hydroxide
- Synonyms
- RbOH;Rubidium hydoxide;RUBIDIUM HYDROXIDE;rubidiumhydroxide(rb(oh));Rubidium hydroxide (Rb(OH));Rubidium hydroxide 1310-82-3;RUBIDIUM HYDROXIDE MONOHYDRATE;Rubidium Hydroxide, 50 % solution;RUBIDIUM HYDROXIDE 99.9% 50% AQ. SOLN.;RUBIDIUM HYDROXIDE, MONOHYDRATE, 99.8%
- CBNumber:
- CB4233581
- Molecular Formula:
- HORb
- Molecular Weight:
- 102.48
- MDL Number:
- MFCD00011194
- MOL File:
- 1310-82-3.mol
| Melting point | 300-302°C |
|---|---|
| Density | 1.74 g/mL at 25 °C |
| solubility | soluble in ethanol |
| form | Liquid |
| color | Pale yellow |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,8285 |
| CAS DataBase Reference | 1310-82-3(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 2692362HBM |
| NIST Chemistry Reference | Rubidium monohydroxide(1310-82-3) |
| EPA Substance Registry System | Rubidium hydroxide (Rb(OH)) (1310-82-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302+H312+H332-H314 | |||||||||
| Precautionary statements | P261-P280-P301+P312-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | C | |||||||||
| Risk Statements | 20/21/22-34-22 | |||||||||
| Safety Statements | 26-27-28-36/37/39-45 | |||||||||
| RIDADR | UN 2677 8/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | VL8750000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28259085 | |||||||||
| NFPA 704 |
|
Rubidium hydroxide price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 243698 | Rubidium hydroxide solution 50 wt. % in H2O, 99% trace metals basis | 1310-82-3 | 25g | $282 | 2024-03-01 | Buy |
| Sigma-Aldrich | 243698 | Rubidium hydroxide solution 50 wt. % in H2O, 99% trace metals basis | 1310-82-3 | 5g | $86.8 | 2023-06-20 | Buy |
| Alfa Aesar | 010565 | Rubidiumhydroxide,50%w/waq.soln. 99.6+%(metalsbasis) | 1310-82-3 | 10g | $141 | 2024-03-01 | Buy |
| Alfa Aesar | 010565 | Rubidium hydroxide, 50% w/w aq. soln., 99.6+% (metals basis) | 1310-82-3 | (c)10g | $136 | 2023-06-20 | Buy |
| Alfa Aesar | 010565 | Rubidium hydroxide, 50% w/w aq. soln., 99.6+% (metals basis) | 1310-82-3 | (c)50g | $645 | 2023-06-20 | Buy |
Rubidium hydroxide Chemical Properties,Uses,Production
Chemical Properties
clear colorless solution
Physical properties
Grayish-white orthogonal crystals; hygroscopic; density 3.2 g/cm3; melts at 301°C; very soluble in water (100 g/100 mL at 15°C), the solution highly alkaline; soluble in ethanol.
Uses
Rubidium hydroxide is an important intermediate in the synthesis of other rubidium compounds and can be used as a catalyst in oxidative chlorination reactions. It is also used in photography and storage batteries. Adding Rubidium hydroxide to fireworks to give it a violet color.
Definition
ChEBI: Rubidium hydroxide is an alkali metal hydroxide and a rubidium molecular entity.
Preparation
Rubidium hydroxide may be obtained as an intermediate in recovering rubidium metal from mineral lepidolite (see Rubidium). In the laboratory it may be prepared by adding barium hydroxide to a solution of rubidium sulfate. The insoluble barium sulfate is separated by filtration:
Rb2SO4 + Ba(OH)2 → 2RbOH + BaSO4
Preparation should be in nickel or silver containers because rubidium hydroxide attacks glass. The solution is concentrated by partial evaporation. The commercial product is usually a 50% aqueous solution.
General Description
A clear aqueous solution. Very caustic. Very irritating to skin and eyes. Used in electric storage batteries.
Air & Water Reactions
Absorbs carbon dioxide from the air. May generate large amounts of heat when diluted with water.
Reactivity Profile
A strong base. Forms caustic solution in water [Merck 11th ed. 1989].
Hazard
Strong irritant to tissue.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
Moderately toxic by ingestion. A powerful, corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of RbzO. See also POTASSIUM HYDROXIDE and RUBIDIUM.
Rubidium hydroxide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Huaran Industrial Co.,Ltd | 17749774353 | 1715438216@qq.com | China | 52 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | eric@witopchemical.com | China | 23556 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
View Lastest Price from Rubidium hydroxide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-11-13 | Rubidium hydroxide
1310-82-3
|
US $0.00 / kg | 1kg | 0.99 | 20 tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-09-06 | Rubidium hydroxide
1310-82-3
|
US $0.00-0.00 / KG | 1KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. | |
 |
2020-02-20 | Rubidium hydroxide
1310-82-3
|
US $1.00 / KG | 1KG | 99% | 200kgs | Career Henan Chemical Co |
-

- Rubidium hydroxide
1310-82-3
- US $0.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.
-

- Rubidium hydroxide
1310-82-3
- US $0.00-0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
-

- Rubidium hydroxide
1310-82-3
- US $1.00 / KG
- 99%
- Career Henan Chemical Co