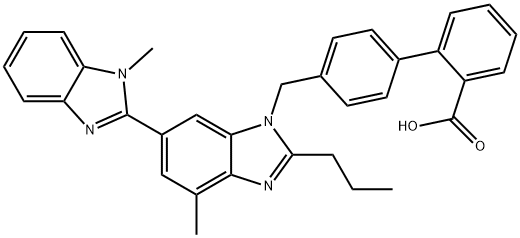
Telmisartan
- CAS No.
- 144701-48-4
- Chemical Name:
- Telmisartan
- Synonyms
- MICARDIS;TU-199;TIMISHATAN;Telmisaran;2-(4-{[4-Methyl-6-(1-Methyl-1H-1,3-benzodiazol-2-yl)-2-propyl-1H-1,3-benzodiazol-1-yl]Methyl}phenyl)benzoic acid;Telmisartan,4′[(1,4′-Dimethyl-2′-propyl[2,6′-bi-1H-benzimidazol]-1′-yl)methyl][1,1′-biphenyl]-2-carboxylic acid, BIBR 277;PRITOR;BIBR 277;misartan;BIBR 277SE
- CBNumber:
- CB4266172
- Molecular Formula:
- C33H30N4O2
- Molecular Weight:
- 514.62
- MDL Number:
- MFCD00918125
- MOL File:
- 144701-48-4.mol
- MSDS File:
- SDS
| Melting point | 261-263°C |
|---|---|
| Boiling point | 771.9±70.0 °C(Predicted) |
| Density | 1.16 |
| RTECS | DV2037500 |
| storage temp. | 2-8°C |
| solubility | DMSO: >5 mg/mL at 60 °C |
| pka | 3.86±0.36(Predicted) |
| form | solid |
| color | white |
| Water Solubility | insoluble |
| Merck | 14,9129 |
| BCS Class | 2 |
| Stability | Hygroscopic |
| InChIKey | RMMXLENWKUUMAY-UHFFFAOYSA-N |
| SMILES | C1(C2=CC=C(CN3C(CCC)=NC4=C(C)C=C(C5N(C)C6=CC=CC=C6N=5)C=C43)C=C2)=CC=CC=C1C(O)=O |
| CAS DataBase Reference | 144701-48-4(CAS DataBase Reference) |
| FDA UNII | U5SYW473RQ |
| ATC code | C09CA07 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P362+P364-P403+P233-P501c |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 22-24/25-36-26 |
| WGK Germany | 2 |
| HS Code | 2933995300 |
Telmisartan price More Price(53)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHR1855 | Telmisartan Pharmaceutical Secondary Standard; Certified Reference Material | 144701-48-4 | 500mg | $214 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1643419 | Telmisartan United States Pharmacopeia (USP) Reference Standard | 144701-48-4 | 200mg | $436 | 2024-03-01 | Buy |
| TCI Chemical | T2861 | Telmisartan >98.0%(HPLC)(T) | 144701-48-4 | 1g | $144 | 2024-03-01 | Buy |
| TCI Chemical | T2861 | Telmisartan >98.0%(HPLC)(T) | 144701-48-4 | 5g | $358 | 2024-03-01 | Buy |
| Alfa Aesar | J61441 | Telmisartan, 99% | 144701-48-4 | 250mg | $69.8 | 2023-06-20 | Buy |
Telmisartan Chemical Properties,Uses,Production
Chemical Properties
White or off-white crystalline powder, odorless and tasteless. Soluble in chloroform, slightly soluble in methanol, very slightly soluble in acetone, practically insoluble in water. Slightly soluble in 0.1mol/L hydrochloric acid, soluble in 0.1mol/L sodium hydroxide.
Absorption coefficient(E1%1cm):509~541
Mechanisms of Action
80mg of telmisartan for the human body will practically completely counter the high blood pressure caused by angiotension II. Effects will last for 24 and can still be detected within 48 hours. Blood pressure-lowering effects should gradually become apparent within 3 hours after the initial dose. If treatment is suddenly interrupted, blood pressure will return to the same level as before treatment in a matter of days, and there will be no rebound high blood pressure. In a clinical trial that compared two kinds of antihypertensive drugs, patients in the treatment group experienced lower rates of coughing than those in the group treated by angiotensin converting enzyme inhibitors.
Telmisartan has a half-life of 18~24 hours, and will take effect 1~4 hours after taken. Medicinal effects can last for as long as 35 hours, with a high (T/P) ratio and outstanding effects in controlling morning blood pressure. Thus, this medicine can effectively control blood pressure for 24 hours, and it meets the once-daily medicinal standard (40~80mg, qd).
Clinical effects and characteristics:
- Pharmacokinetics show: Rapid effects (0.3h), long duration (35.4h), little effect on heart rate when lowering blood pressure.
- Compared to Enalapril: Stronger antihypertensive effects than Enalapril; when both are used in combination with diuretics, Telmisartan still appears to be superior and results in a lower coughing rate.
- Compared to Lisinopril: More apparent antihypertensive effects (for both systolic and diastolic blood pressure), coughing rate for Telmisartan (16%) is drastically lower than that of Lisinopril (60%).
- Compared to Atenolol: Similar antihypertensive effects, lower rate of side effects (impotence and fatigue).
- Compared to Amlodipine: The Telmisartan group experienced noticeably lower heart rates four hours after ingestion and from six a.m. to twelve p.m.
Side Effects
In the placebo control experiment, the incidence rate of negative events for the Telmisartan group (41.4%) was similar to that of the placebo group (43.9%). Negative events were unrelated to dosage, sex, age, or race.
The following lists negative reactions were accumulated via the 5788 hypertensive patients that were treated with Telmisartan in the clinical trial.
Negative effects are categorized by incident rate as:
Very common (>1/10); common (>1/100, <1/10=); uncommon (>1/1000, <1/100=); rare (>1/10000, <1/1000=); very rare (<1/100000=)
Bodily reaction: Common: Back pain (e.g. sciatica), chest pain, flu-like symptoms, infection symptoms (e.g. urinary tract infection including cystitis). Uncommon: sight abnormality, sweating.
Central and peripheral nervous system: Common: vertigo.
Digestive system: Common: stomach pain, diarrhea, indigestion, gastrointestinal disorders. Rare: dry mouth, bloating.
Muscle skeletal system: Common: joint pain, leg cramps or leg pain, muscle pain. Rare: Tenosensitis symptoms.
Nervous system: Rare: anxiety.
Respiratory system: Common: upper respiratory tract infection, including pharyngitis and rhinitis.
Skin and accessory system: Common: Skin abnormalities such as eczema.
Additionally, since Telmisartan entered the market, there have been individual cases of erythema, itching, syncope, insomnia, depression, stomach discomfort, vomiting, hypotension, bradycardia, tachycardia, dyspnea, eosinophilia, thrombocytopenia, weakness, and reduced work efficiency. Similar to other angiotensin II receptor blockers, very few cases have reported vascular edema, nettles, and other negative reactions.
The laboratory found: compared to the placebo, the Telmisartan group occasionally exhibited lowered hemoglobin or raised uric acid. Any increase in serum creatinine or liver enzymes in the Telmisartan group was similar or lower than that of the placebo group.
Patent
Telmisartan was originally formulated by the German pharmaceutical company Boehringer Ingelheim; it earned the German patent EP502,314 in 1991, was first approved to enter the American market in November 1998, and then entered German, Philippines, Australian, Belgium, British, and other markets.
Description
Telmisartan is an angiotensin receptor blocker (ARB), It is used in the treatment of hypertension also effective in cardiovascular risk reduction.Telmisartan blocks the action of angiotensin II (Ang II), the primary effector molecule of the renin-angiotensin-aldosterone system (RAAS). It is the sixth of this class of 《sartans》 to be marketed after the lead compound Losartan. Its long lasting effect (24h half-life) could be the main difference with other angiotensin II antagonists. Unlike several other agents in this category, its activity does not depend upon transformation into an active metabolite, the 1-O-acylglucuronide being the principal metabolite found in humans. Telmisartan is a potent competitive antagonist of AT1 receptors that mediate most of the important effects of angiotensin II while lacking affinity for the AT2 subtypes or other receptors involved in cardiovascular regulation.
Chemical Properties
White or off white crystalline powder
Originator
Boehringer Ingelheim (Germany)
Uses
Telmisartan, an angiotensin II receptor antagonist, is an effective medication for the treatment of hypertension. It can be used alone or in combination with other antihypertensive drugs. Additionally, it is beneficial in the treatment of diabetic nephropathy in hypertensive individuals with type 2 diabetes mellitus. Telmisartan is also used to address congestive heart issues.
Definition
ChEBI: Telmisartan is a member of the class of benzimidazoles used widely in the treatment of hypertension. It has a role as an antihypertensive agent, an angiotensin receptor antagonist, an EC 3.4.15.1 (peptidyl-dipeptidase A) inhibitor, a xenobiotic and an environmental contaminant. It is a member of biphenyls, a member of benzimidazoles and a carboxybiphenyl.
Manufacturing Process
Telmisartan was synthesized through the reaction of methyl 3,4-diaminobenzoate dihydrochloride and butyric acid chloride in the presence of phosphorous oxychloride, with subsequent purification steps:
A solution of 23.9 g (100 mMol) of methyl 3,4-diaminobenzoate
dihydrochloride and 11.7 g (110 mMol) of butyric acid chloride in 100 ml of
phosphorus oxychloride is refluxed for 2 h. Then about 80 ml of phosphorus
oxychloride are distilled off and the residue is mixed with about 150 ml of
water. The oily crude product precipitated is extracted three times with 50 ml
of ethyl acetate and after evaporation purified by column chromatography
(600 g of silica gel; eluant:methylene chloride/methanol (30:1)). Yield of
methyl-2-n-propyl-benzimidazole-5-carboxylate: 15.0 g of oil (69%).
A solution of 15.0 g (73 mmol) of methyl 2-n-propyl-benzimidazole-5-
carboxylate and 8 g (200 mMol) of sodium hydroxide in 200 ml of water and
400 ml of ethanol is refluxed for 2 h. Then the alcohol is distilled off, the
aqueous solution is acidified with dilute sulphuric acid (pH 4-5) and
evaporated using a rotary evaporator. The product crystallising out is suction filtered, washed with 50 ml of acetone and 50 ml of diethylether and dried.
Yield of 2-n-propyl-benzimidazole-5-carboxylic acid-hemisulphate: 9.1 g
(61%), melting point: >220°C.
A solution of 6.7 g (25 mMol) of 2-n-propyl-benzimidazole-5-carboxylic acidhemisulphate
and 4.9 g (25 mMol) of 2-methylaminoaniline dihydrochloride in
200 g of polyphosphoric acid is stirred for 5 h at 150°C, then poured onto 600
ml of water and made alkaline with concentrated ammonia whilst cooling with
ice. The resulting solution is extracted three times with 200 ml of ethyl
acetate, the crude product thus obtained is purified by column
chromatography (300 g of silica gel; eluant:methylene chloride/methanol =
15:1). Yield of 2-n-propy1-5-(1-methylbenzimidazol-2-yl)-benzimidazole: 2.8
g of oil (39%).
A solution of 2.0 g (6.9 mMol) of 2-n-propyl-5-(1-methylbenzimidazol-2-yl)-
benzimidazole and 0.91 g (7.5 mmol) of potassium tert-butoxide in 50 ml of
dimethylsulfoxide is stirred for 90 min at room temperature, then 2.6 g (7.5
mMol) of tert-butyl 4'-bromomethyl-biphenyl-2-carboxylate are added and the
mixture is stirred for a further 15 h at room temperature. The mixture is then
poured onto 300 ml of water and extracted three times with 50 ml of ethyl
acetate. The crude product obtained after evaporation of the organic phase is
purified by column chromatography (300 g silica gel; eluant:methylene
chloride/methanol = 30:1). In this way, 2.7 g (70%) of an isomer mixture are
obtained (by NMR spectroscopy), contains about 1.18 g of tert-butyl-4'-[(2-npropyl-
5-(1-methylbenzimidazol-2-yl)-benzimidazol-1-yl)-methyl]biphenyl-2-
carboxylate and about 1.52 g of tert-butyl 4'-[(2-n-propyl-6-(1-
methylbenzimidazol-2-yl)-benzimidazol-1-yl)-methyl]biphenyl-2-carboxylate).
2.70 g of the isomer mixture obtained above are dissolved in 100 ml of
methylene chloride, mixed with 40 ml of trifluoroacetic acid and stirred for 4 h
at room temperature. The mixture is then evaporated to dryness in vacuo, the
residue is dissolved in 100 ml of 2 N sodium hydroxide solution, the solution
is washed with 50 ml of diethylether and the product mixture is precipitated
by acidifying the aqueous phase with acetic acid. By column chromatography
(400 g of silica gel, eluant:methylene chloride/methanol = 15:1) of the solid
thus obtained 0.9 g (74%) of Telmisartan, melting point 217°-218°C.
brand name
Micardis (Boehringer Ingelheim).
Therapeutic Function
Antihypertensive
General Description
Telmisartan, 4'-[(1,4'-dimethyl-2'-propyl[2,6'-bi-1H-benzimidazol]-1'-yl)methyl]-[1,1'-biphenyl]-2-carboxylic acid (Micardis), does not appear to bear any structuralrelationship to this class, but there is actually a great dealof overlap in the chemical architecture with other agents. Thefirst, and most significant, difference is the replacement of theacidic tetrazole system with a simple carboxylic acid. Thisacid, like the tetrazole, plays a role in receptor binding. Thesecond difference is the lack of a carboxylic acid near the imidazolenitrogen that also contributes to receptor binding.As with irbesartan, however, there is not a need for this groupto be acidic but, rather, to be one that participates in receptorbinding. The second imidazole ring, much like a purine basein deoxyribonucleic acid (DNA), can hydrogen bond with theangiotensin II receptor.
Biochem/physiol Actions
Telmisartan is a non-peptide AT1 angiotensin receptor antagonist.
Clinical Use
Angiotensin-II antagonist:
Hypertension
Prevention of cardiovascular events
Synthesis
Telmisartan can be prepared in eight steps starting with methyl 4-amino-3-methyl benzoate; the first and second cyclization into a benzimidazole ring occur at steps 4 and 6 respectively.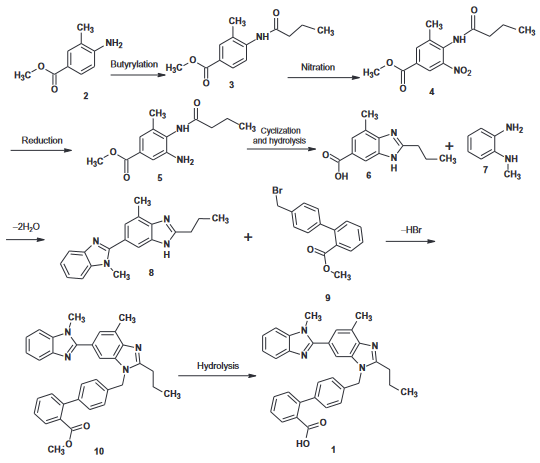
Drug interactions
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: antagonism of hypotensive effect and
increased risk of renal impairment with NSAIDs;
hyperkalaemia with ketorolac and other NSAIDs.
Antihypertensives: increased risk of hyperkalaemia,
hypotension and renal impairment with ACE-Is and
aliskiren.
Cardiac glycosides: concentration of digoxin
increased.
Ciclosporin: increased risk of hyperkalaemia and
nephrotoxicity.
Diuretics: enhanced hypotensive effect;
hyperkalaemia with potassium-sparing diuretics.
ESAs: increased risk of hyperkalaemia; antagonism
of hypotensive effect.
Lithium: reduced excretion (possibility of enhanced
lithium toxicity).
Potassium salts: increased risk of hyperkalaemia.
Tacrolimus: increased risk of hyperkalaemia and
nephrotoxicity.
Metabolism
Telmisartan is metabolised by conjugation to the
glucuronide of the parent compound. No pharmacological
activity has been shown for the conjugate.
Telmisartan is excreted almost entirely in the faeces via
bile, mainly as unchanged drug.
Clinical claims and research
In several clinical studies, Telmisartan, at a once daily dosage, produced effective and sustained blood-pressure lowering effects with a low incidence of side effects (particularly treatment-related cough associated with ACE inhibitors in elderly patients).
Telmisartan Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| shan dong Fengjin Pharmaceutical company | +8615066764791 | liangfulin@fengjin-pharma.com | China | 8 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5892 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 10991 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 994 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20291 | 58 |
| Sigma Audley | +86-15937194204 +86-18126314766 | nova@sh-teruiop.com | China | 491 | 58 |
| Hangzhou Hyper Chemicals Limited | +86-0086-57187702781 +8613675893055 | info@hyper-chem.com | China | 295 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 | sarah@tnjone.com | China | 1143 | 58 |
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +8615383190639 | admin@86-ss.com | China | 1000 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29798 | 60 |
Related articles
- Telmisartan: The 'patron saint' of Hypertension Management
- This paper discuss the pharmacological mechanism, clinical application, adverse reactions, drug interactions, and future devel....
- Jun 25,2024
- What side effects can Telmisartan cause?
- Telmisartan (Micardis) is an angiotensin receptor blocker (ARB). This type of medication blocks a chemical in your body that n....
- May 15,2023
- Telmisartan: uses & side-effects
- Telmisartan oral tablet is a prescription drug that’s available as the brand-name drug Micardis. It’s also available as a gene....
- Aug 19,2019
Related Qustion
- Q:Is telmisartan the same as losartan?
- A:The most often prescribed ARBs are telmisartan and losartan. Both drugs are ARBs. However, they are different.
- Jun 11,2024
View Lastest Price from Telmisartan manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-24 | Telmisartan
144701-48-4
|
US $999.00-666.00 / kg | 1kg | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-09-24 | Telmisartan
144701-48-4
|
US $0.00 / Kg/Bag | 2Kg/Bag | EP / USP | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-09-23 | Telmisartan
144701-48-4
|
US $10.50 / KG | 1KG | 99% | 10 ton | Hebei Chuanghai Biotechnology Co,.LTD |
-

- Telmisartan
144701-48-4
- US $999.00-666.00 / kg
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- Telmisartan
144701-48-4
- US $0.00 / Kg/Bag
- EP / USP
- Sinoway Industrial co., ltd.
-

- Telmisartan
144701-48-4
- US $10.50 / KG
- 99%
- Hebei Chuanghai Biotechnology Co,.LTD
144701-48-4(Telmisartan)Related Search:
1of4