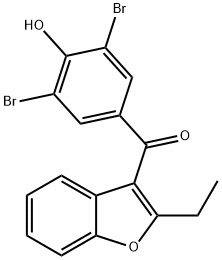
Benzbromarone
- CAS No.
- 3562-84-3
- Chemical Name:
- Benzbromarone
- Synonyms
- l2214;desuric;exurate;minuric;mj10061;Besuric;Urinorm;Normurat;l2214-labaz;AzubroMaron
- CBNumber:
- CB4408363
- Molecular Formula:
- C17H12Br2O3
- Molecular Weight:
- 424.08
- MDL Number:
- MFCD00078962
- MOL File:
- 3562-84-3.mol
| Melting point | 151° |
|---|---|
| Boiling point | 514.1±50.0 °C(Predicted) |
| Density | 1.6211 (rough estimate) |
| refractive index | 1.6010 (estimate) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Practically insoluble in water, freely soluble in acetone and in methylene chloride, sparingly soluble in ethanol (96 per cent). |
| pka | 4.66±0.25(Predicted) |
| color | White to Light yellow |
| Merck | 14,1065 |
| InChIKey | WHQCHUCQKNIQEC-UHFFFAOYSA-N |
| CAS DataBase Reference | 3562-84-3(CAS DataBase Reference) |
| FDA UNII | 4POG0RL69O |
| ATC code | M04AB03 |
| EPA Substance Registry System | Methanone, (3,5-dibromo-4-hydroxyphenyl)(2-ethyl-3-benzofuranyl)- (3562-84-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301 | |||||||||
| Precautionary statements | P301+P310 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22 | |||||||||
| Safety Statements | 36 | |||||||||
| RIDADR | UN 2811 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | OB1804200 | |||||||||
| HS Code | 2932.99.7000 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| Toxicity | LD50 oral in rat: 248mg/kg | |||||||||
| NFPA 704 |
|
Benzbromarone price More Price(31)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | B5774 | Benzbromarone analytical standard | 3562-84-3 | 1g | $72.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 09147 | Benzbromarone certified reference material, TraceCERT | 3562-84-3 | 50mg | $109 | 2024-03-01 | Buy |
| TCI Chemical | B4099 | Benzbromarone >98.0%(GC)(T) | 3562-84-3 | 1g | $56 | 2024-03-01 | Buy |
| TCI Chemical | B4099 | Benzbromarone >98.0%(GC)(T) | 3562-84-3 | 5g | $186 | 2024-03-01 | Buy |
| Cayman Chemical | 19768 | Benzbromarone ≥98% | 3562-84-3 | 1g | $44 | 2024-03-01 | Buy |
Benzbromarone Chemical Properties,Uses,Production
Description
Benzbromarone,a non-competitive inhibitor of xanthine oxidase,is apotent uricosuric drug used in the treatment of gout.
Benzbromarone is a benzofuran derivative chemically related to amiodarone. It increases uric acid excretion by non-specificallyinhibiting its tubular reabsorption.
lt is used in patients with venous disorders to prevent,retard,or reverse varicose degenerative changes in the vessel wall.
Benzbromarone causes diarrhea (3-4% of patients), urate and oxalate stones,urinary sand,renal colic,and allergy in a small number of patients.Liver damage, which reverses after withdrawal, has been described (SEDA-18,108).
Chemical Properties
Pale Beige Solid
Originator
Desuric,Labaz,Switz.
Uses
Coronarodilatator;Uric acid transport inhibitor
Uses
Benzbromarone is a benzofuran derivative that has been reported to lower serum urate levels in animals and human studies. In normal and hyperuricaemic subjects, benzbromarone reduced serum uric acid levels by one-third to one-half. In comparison with other urate-lowering drugs, 80 mg of micronized or 100 mg of nonmicronized benzbromarone had equal urate-lowering activity to 1–1.5 g of probenecid or 400–800 mg of sulfinpyrazone.
The mechanism of the urate-lowering activity of benzbromarone appears to be attributable to its uricosuric activity. In rats, benzobromarone inhibited urate reabsorption in the proximal tubules when given at 10 mg/kg i.v. In isolated rat liver preparation, benzbromarone inhibits xanthine oxidase in vitro but not in vivo . In humans, this compound only weakly inhibits xanthine oxidase and no increase in urinary excretion of xanthine or hypoxanthine was observed. After oral administration, about 50% of benzbromarone is absorbed. The drug undergoes extensive dehalogenation in the liver and is excreted mainly in the bile and feces. For control of gout the usual therapeutic dose is 100–200 mg daily. Benzbromarone has few side effects and is usually well tolerated.
Uses
Benzbromarone is a uricosuric agent used in the treatment of gout and hyperuricemia. Studies show that use of Benzbromarone results in less complications than other uricosuric agent such as Allopurinol.
Definition
ChEBI: Benzbromarone is 1-Benzofuran substituted at C-2 and C-3 by an ethyl group and a 3,5-dibromo-4-hydroxybenzoyl group respectively. An inhibitor of CYP2C9, it is used as an anti-gout medication. It has a role as a uricosuric drug. It is a member of 1-benzofurans and an aromatic ketone. It derives from a 2,6-dibromophenol.
Manufacturing Process
The propyl analog of the benzbromarone intermediate containing an ethyl
group is prepared as follows: to a solution of potassium hydroxide (56 g = 1
mol) in absolute ethyl alcohol (750 cc) is added one mol of salicylic aldehyde
(122 grams). The mixture is brought to boiling point in a water-bath until the
potassium salt formed is dissolved. One mol of ethyl chloromethyl ketone
(106.5 grams) (methyl chloromethyl ketone or chloracetone in the case of
benzbromarone) is gradually added and the solution boiled in a reflux
condenser for two hours.
After cooling, the potassium chloride precipitate is separated off by filtration,
and the greater part of the solvent removed by distillation. The residue is then
puritied by distillation. In this way, 140 grams of 2-propionyl coumarone are
obtained, boiling at 135°C under 15 mm Hg. A mixture is then prepared as
follows: 215 grams of 2-propionyl coumarone, 550 cc of diethylene glycol and
200 grams of hydrazine hydrate at 85% and maintained at boiling point in a
reflux condenser for 10 minutes. After cooling, 180 grams of potassium
hydroxide are added and the mixture brought up to 120°-130°C. This
temperature is maintained until no more nitrogen is liberated (about 1 hour).
The mixture is then distilled by means of super-heated steam (150°-160°C).
The distillate is neutralized by means of concentrated HCl, decanted, and the
aqueous layer extracted by means of ether. The oily layer and the ethereal
extract are mixed, washed with diluted HCl, then with water, and finally dried
over sodium sulfate. The solvent is removed and the residue rectified under reduced pressure. In this way, 130 grams of 2-propyl coumarone are
obtained, boiling at 112°C under 17 mm of mercury.
The following substances are then placed in a 250 cc flask fitted with a stirrer
and a separatory funnel: 12.96 grams of 2-propyl coumarone, 55 cc of carbon
sulfide and 14 grams of anisoyl chloride. The mixture is cooled by means of
iced water and 21.5 grams of stannic chloride introduced dropwise, while the
mixture is stirred. Stirring is continued for three hours at 0°C, after which the
mixture is allowed to stand overnight. 50 cc of carbon sulfide is added and the
mixture is treated, while being stirred, with the following: 20 cc of HCl and
100 cc of iced water. The organic layer is decanted and washed with water,
dried over silica gel and rectified.
16.16 grams of 2-propyl-3-anisoyl coumarone are obtained (Yield: 72%),
boiling at 189°C under 0.5 mm Hg. The methoxylated coumarone so obtained
is mixed as follows: 1 part of 2-propyl-3-anisoylcoumarone and 2 parts of
pyridine hydrochloride and the mixture maintained for one hour under a
stream of dry nitrogen in an oil bath at 210°C (under a vertical condenser).
After cooling, the mixture is triturated with 0.5 N hydrochloric acid (10 parts).
The aqueous layer is separated and the residue extracted with ether. The
ethereal extract is treated with 20 parts of 1% caustic soda. The alkaline layer
is separated by decanting and acidified by means of diluted HCl. The
precipitate is purified by recrystallization in aqueous acetic acid.
0.8 part of 2-propyl-3p-hydroxybenzoyl coumarone is obtained, melting at
123°C. Then the dibromo counterpart of benzbromarone may be prepared as
follows: 8.05 g of 3-ethyl-2-p-hydroxybenzoyl coumarone, prepared as
described above, are dissolved in very silght excess of 3% caustic soda. To
this solution is gradually added a slight excess of bromine dissolved in a 25%
aqueous solution of potassium bromide. The resultant solution is acidified with
a 20% solution of sodium bisulfite, centrifuged, washed with water and then
dried under vacuum. The product is then recrystallized in acetic acid and 13.6
g of 2-(4'-hydroxy-3',5'-dibromo-benzoyl)-3-ethyl coumarone obtained. MP
151°C.
Therapeutic Function
Uricosuric, Antiarthritic
Safety Profile
Poison by ingestion, intravenous, and intraperitoneal routes. Moderately toxic by subcutaneous route. Experimental teratogenic and reproductive effects. A uricosuric agent which promotesthe excretion of uric acid in the urine. A flammable liquid. When heated to decomposition it emits toxic fumes of Br-. See also KETONES.
Benzbromarone Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-18612256290 | 1056@dideu.com | China | 3632 | 58 |
View Lastest Price from Benzbromarone manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-07-25 | Benzbromarone
3562-84-3
|
US $1400.00 / Kg/Drum | 1KG | 99% | 1000tons/month | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-07-20 | BENZBROMARONE
3562-84-3
|
US $50.00 / kg | 1kg | 99.9% | 10000MT | Henan Bao Enluo International TradeCo.,LTD | |
 |
2023-06-26 | Benzbromarone
3562-84-3
|
US $150.00 / Kg/Bag | 10g | 99.5% | 500kg/month | Hebei Mingeng Biotechnology Co., Ltd |
-

- Benzbromarone
3562-84-3
- US $1400.00 / Kg/Drum
- 99%
- Hebei Yanxi Chemical Co., Ltd.
-

- BENZBROMARONE
3562-84-3
- US $50.00 / kg
- 99.9%
- Henan Bao Enluo International TradeCo.,LTD
-

- Benzbromarone
3562-84-3
- US $150.00 / Kg/Bag
- 99.5%
- Hebei Mingeng Biotechnology Co., Ltd
3562-84-3(Benzbromarone)Related Search:
1of4