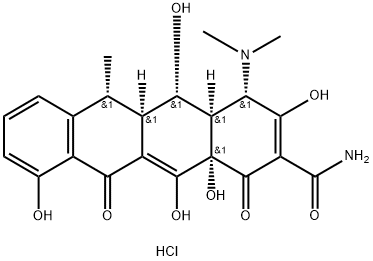
Doxycycline hydrochloride
- CAS No.
- 10592-13-9
- Chemical Name:
- Doxycycline hydrochloride
- Synonyms
- DOXYCYCLINE HCL;Hyclate;Doxycycline Hydrochloride, Ready Made Solution;DOXYCYCLINE HYDROCHLORIDE HEMIETHANOLATE HEMIHYDRATE;ecodox;retens;doxy-ii;samecin;tecacin;midoxin
- CBNumber:
- CB4489445
- Molecular Formula:
- C22H25ClN2O8
- Molecular Weight:
- 480.9
- MDL Number:
- MFCD03427564
- MOL File:
- 10592-13-9.mol
- MSDS File:
- SDS
| Melting point | 195-201℃ |
|---|---|
| Flash point | 87℃ |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | H2O: 50 mg/mL, clear, yellow-green |
| form | powder |
| color | yellow |
| Water Solubility | Soluble in water. |
| BCS Class | 1 |
| InChIKey | RUYHIJHUVHIMIR-PGTYNCOMNA-N |
| SMILES | O[C@@]12C(C(C(=O)N)=C(O)[C@@H](N(C)C)[C@]1([H])[C@H]([C@]1([H])[C@H](C3C=CC=C(O)C=3C(=O)C1=C2O)C)O)=O.Cl |&1:1,9,13,15,16,18,r| |
| CAS DataBase Reference | 10592-13-9(CAS DataBase Reference) |
| FDA UNII | 4182Z6T2ET |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H361d |
| Precautionary statements | P202-P264-P270-P280-P301+P312-P308+P313 |
| Hazard Codes | Xn,Xi |
| Risk Statements | 22-36/37/38 |
| Safety Statements | 26-36/37 |
| RIDADR | NA 1993 / PGIII |
| WGK Germany | 3 |
| F | 8 |
| HS Code | 29413000 |
Doxycycline hydrochloride price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | D3447 | Doxycycline hydrochloride | 10592-13-9 | 500mg | $74.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | D3072 | Doxycycline Hydrochloride, Ready Made Solution | 10592-13-9 | 1ml | $52.8 | 2024-03-01 | Buy |
| Alfa Aesar | J60422 | Doxycycline hydrochloride | 10592-13-9 | 5g | $102 | 2024-03-01 | Buy |
| Alfa Aesar | J60422 | Doxycycline hydrochloride | 10592-13-9 | 25g | $398 | 2024-03-01 | Buy |
| Alfa Aesar | J67043 | Doxycycline hydrochloride, 20 mg/ml in distilled water, sterile-filtered | 10592-13-9 | 50ml | $143 | 2024-03-01 | Buy |
Doxycycline hydrochloride Chemical Properties,Uses,Production
Overview
Doxycycline hydrochloride is the hydrochloride form of doxycycline, being a tetracycline antibiotic that has enjoyed widespread use in both veterinary and human medicine owing to its relatively broad spectrum and wide margin of safety. The first members of the tetracycline class were isolated from several species of bacteria from the genus Streptomyces in the1940s and 1950s. Since that time, a variety of tetracycline have been discovered, both naturally produced (e.g., chlortetracycline) and semisynthetic (e.g., doxycycline and tetracycline). Doxycycline was discovered in 1967 and has undergone extensive investigation, both for its antimicrobial properties as well as the effects it has on the physiology of higher organisms[1].
Globally, doxycycline has remained one of the most commonly used and inexpensive of the broad-spectrum antibiotic drugs currently in use[2]. In Australia, over the period 2002–2005, there were still well over 800,000 prescriptions of doxycycline 100 mg annually[3]. The development of doxycycline in 1967 followed the development of tetracycline class of compounds and their clinical application in 1953[4]. It has also been observed that it has been prescribed up to three times as often as minocycline, another commonly used drug in the tetracycline class[4]. Doxycycline has a variety of applications, but it is known that the tetracycline class of antibiotics does have a range of side-effects and noteworthy contraindications[2].
Two different commercial doxycycline products are available for use in dogs; doxycycline hyclate tablets (Ronaxan; Merial) are available in several European countries, and doxycycline monohydrate paste or tablets (VibraVet; Pfizer) are available in other countries, including Australia, New Zealand, and South Africa. These products are used for treatment of respiratory tract infections and other infections caused by a variety of bacteria, including staphylococci.
Origin and structure
The development of tetracycline antibiotics was the result of a systematic screening of soil specimens collected from many parts of the world for antibiotic-producing organisms. A number of tetracyclines exist, all very much alike in structure and function. Tetracycline and doxycycline are semi-synthetically produced from a species of Streptomyces and differ only by the position of a single hydroxy moiety on carbon#5.[5]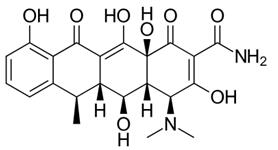
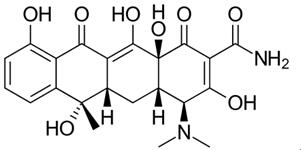
Figure 1 the chemical structure of doxycycline and tetracycline.
Applications and indication
Doxycycline has a significant application in the treatment of common chronic conditions, such as acne and rosacea; however its use in a range of more unusual infectious diseases, including what Holmes et al describe as “atypical bacteria”, has given doxycycline some fame as a "wonder drug" or the "secret weapon of the infectious disease physician".[2] Besides its treatment of common causes of respiratory and genitourinary tract infections, some of its broader applications are again diseases such as rickettsial infections, leptospirosis, malaria, brucellosis, and a number of sexually transmitted infections[2] should not be underestimated. It also has a variety of dental applications[6-11]. There was also a 30% increase in the number of prescriptions following the anthrax bioterrorism scares in 2000–2001.10 In addition to anthrax, doxycycline could have application in the event of other bioterrorist agents being used, such as tularaemia and the plague.1 Future applications may also involve the treatment of some parasitic infections, such as lymphatic filariasis, where it appears to have action against the endosymbiotic bacteria of certain filariae.[11]
It is used in the treatment of infections[12] caused by various pathogens including susceptible Rickettsia, Chlamydia, Chlamydophila, and Mycoplasma; malaria prophylaxis (areas with chloroquineor pyrimethamine-sulfadoxine resistant strains) for short-term travel (<4 months); treatment for syphilis, uncomplicated Neisseria gonorrhoeae (alternative agent), Listeria, Actinomyces israelii, and Clostridium infections in penicillin-allergic patients; used for community-acquired pneumonia and other common infections due to susceptible organisms; anthrax due to Bacillus anthracis, including inhalational anthrax (postexposure); treatment of infections caused by uncommon susceptible gram-negative and gram-positive organisms including Borrelia recurrentis, Ureaplasma urealyticum, Haemophilus ducreyi, Yersinia pestis, Francisella tularensis, Vibrio cholerae, Campylobacter fetus, Brucella spp, Bartonella bacilliformis, and Klebsiella granulomatis, Q fever, Lyme disease; intestinal amebiasis; severe acne.
Doxycycline has probably been the most widely used antimalarial in Australia over the past 15 years[3, 13, 14]. Although its application as a malaria chemoprophylaxis varies from country to country,[3] it was likely to be the most common antimalarial used in New Zealand[15] and South Africa,[16] although in each case it is hard to quantify as doxycycline has indications other than malaria prophylaxis. It is useful as it provides effective antimalarial prophylaxis in areas with high levels of transmission of mefloquine-resistant or multidrug-resistant malaria due to Plasmodium falciparum,[11] although there are concerns that it may not be adequate prophylaxis for P. vivax[11]. In more recent trials using the currently recommended dosage (100 mg/day as the adult dose), doxycycline has shown protective efficacy ranging from 92%–100% in non-immune and semi-immune adults living in endemic areas[9]. In areas of chloroquine-resistant malaria, it was equivalent to mefloquine and atovaquone/proguanil and superior to chloroquine/proguanil[17]. Resistance has not been reported among Plasmodium spp, although bacterial resistance occurs,[18] as will be discussed under efficacy.
Pharmacodynamics
Tetracycline is absorbed incompletely from the gastrointestinal tract; only 60% is absorbed under optimal conditions. Plasma concentrations of doxycycline, however, essentially are equivalent whether given orally or intravenously because the drug’s absorption is nearly complete. The absorption of both drugs is much greater in the fasting state and is impaired substantially by food, milk products, antacids, calcium, and iron preparations. Tetracycline is affected much more by these factors than doxycycline. Both tetracycline and doxycycline should be taken 3 hours before, or 2 hours after, taking iron or antacids, and tetracycline should not be taken with meals.
Once in the serum, the half-lives of tetracycline and doxycycline are 6-1.2 hours and 16-18 hours, respectively. Hence, tetracycline requires four-times-a-day dosing, whereas doxycycline only needs to be taken twice daily[19, 20].
The volume of distribution of tetracyclines includes not only that of the extracellular serous fluid but the tears, saliva, cerebrospinal fluid, and amniotic fluid. Concentrations in these secretions and fluids are only IO-25% of those found in the serum, and only doxycycline is known to accumulate in the cerebrospinal fluid. All tetracyclines are concentrated in the liver and excreted by way of the bile into the intestine. Because of the effects of the enterohepatic circulation, the tetracyclines are excreted and resorbed continually and thus may be present in the blood for a long time after cessation of therapy. Tetracycline is eliminated primarily through the kidney. Doxycycline, on the other hand, is eliminated almost exclusively in the feces and is, therefore, one of the safest of the antibiotics in the presence of renal failure[5, 20].
Mode of action
The site of action of tetracycline and doxycycline is the bacterial ribosome.
The drugs bind primarily to 30 S ribosomes and block access of bacterial aminoacyl tRNA to the acceptor sites on mRNA, preventing the addition of amino acids to the growing peptide chain. Resistance is mediated by a plasmid that prevents the intracellular accumulation of tetracyclines. These drugs are primarily bacteriostatic, preventing the organism from reproducing but not killing it outright. Thus, an intact immune system is necessary for eradication of the infection[5, 21]
Adverse reactions and precaution
The most common adverse events described for doxycycline include the oesophageal erosion and photosensitivity (reported as a 10% risk), there were only 130 described between 1966 and 2003 and the denominator is unknown,[4] but it is likely that the number of prescriptions for doxycycline during this period was probably 100’s of millions. There are, however, wider ranges of side effects, which may present with the use of doxycycline, many of which may be reduced by some common sense measures. It should be noted that side effects are not limited to medical practice and may be seen in dental practice as well.[24] Some of the more unusual side effects include photo-onycholysis, various skin eruptions, Stevens-Johnson syndrome, Jarisch-Herxheimer reaction, and benign intracranial hypertension, as well as a potential risk of hepatotoxicity, which is thought to be low compared to other tetracyclines.[2]
Doxycycline should not be used by pregnant or lactating women or by children younger than 8 years because of its deleterious effects on bone and tooth development.[2] Also, it should not be used by persons with known allergy or sensitivity to the drug.[4, 22] Some more tips are summarized below:
- Take with a meal
- Do not lie down for at least 30 minutes to 1 hour after taking the drug to prevent its reflux into the esophagus resulting in esophagitis
- Swallowed with a full glass of water to ensure that it does not get stuck in the esophagus, leading to esophageal ulceration
- Women using doxycycline should be recommended to obtain an over-the counter antifungal drug or prescribed an antifungal drug to treat vaginal candidiasis, if they become symptomatic
- Avoid peak (midday) sun exposure
- Use a sunscreen preparation that contains an ultraviolet A (UVA) blocker since the phototoxicity associated with doxycycline is UVA induced.
References
- Papich MG, Riviere JE: Tetracycline antibiotics, in Riviere JE, Papich MG, Adams HR (eds): Veterinary Pharmacology and Therapeutics (ed 9). Ames, IA, Wiley-Blackwell, pp 895-913, 2009
- Holmes NE, Charles PGP. Safety and efficacy review of doxycycline. Clin Med Ther. 2009;1:471–82.
- Leggat PA. Trends in antimalarial prescriptions in Australia 2002–2005. J Travel Med.2008;15:302–6.
- Smith K, Leyden JL. Safety of doxycycline and minocycline: A systemic review. Clin Ther. 2005; 27:1329–42.
- Goodman L, Gilman A. The pharmacologic basis of therapeutics. Macmillan Publishing Co., 1985:1170-8.
- Parola P, Raoult D. Tropical rickettsioses. Clinics Dermatol. 2006; 24: 191–200.
- Terheggan U, Leggat PA. Clinical manifestations of Q fever in adults and children. Travel Med Inf Dis. 2007;5:159–64.
- Guidugli F, Castro AA, Atallah AN. Antibiotics for preventing leptospirosis. Cochrane Database of Systematic Reviews. 2000;4: CD001305.
- Jong EC, Nothdurft HD. Current drugs for antimalarial chemoprophylaxis: A review of efficacy and safety. J Travel Med. 2001;8(Suppl 3):S48–S56.
- Sauret JM, Vilissova N. Human brucellosis. J Am Board Fam Pract. 2002; 15:401–6.
- Mohommadi Z. Local applications of tetracyclines in endodontics and dental trauma: a review. Dent Today. 2009;28:95–6, 98, 100–1, quiz 101.
- http://www.just.edu.jo/DIC/AZLibrary/Doxycycline.pdf
- Leggat PA, Speare R. Trends in antimalarial drugs prescribed in Australia, 1992–1998. J Travel Med. 2003; 10: 189–91.
- Leggat PA. Trends in antimalarial prescription in Australia 1998–2002. J Travel Med. 2005;12: 338–42.
- Leggat PA, Heydon JL. Trends in antimalarial drugs prescribed in New Zealand 1993 to 1998. J Travel Med. 2002;9:156–9.
- Leggat PA, Du?rrheim DN, Blumberg L. Trends in malaria chemoprophylaxis prescription in South Africa 1994–2000. J Travel Med. 2002; 9:318–21.
- Epstein JE, Baird JK, Hoffman SL. Malaria prevention. Curr Treat Options Infect Dis. 2000; 2: 259–65.
- Beallor C, Kain KC. Doxycycline. In: Schlagenhauf P, ed. Travelers’ Malaria. Hamilton, Canada: BC Decker, 2001:210–18.
- Gelman CR, Rumack BH, Hoff AJ, eds. Drug evaluation monograph: doxycycline and tetracycline. Denver (CO): Micromedex Inc., 1985: VoI. 85.
- Neuvonen PJ. Interaction with the absorption of tetracyclines. Drugs 1976; 11: 45-54.
- Barza M, Scheife R. Antimicrobial spectrum, pharmacology, and therapeutic use of antibiotics. J ME Med Assoc 1977; 68:194-212.
- Beallor C, Kain KC. Doxycycline. In: Schlagenhauf P, ed. Travelers’ Malaria. Hamilton, Canada: BC Decker, 2001:210–18.
- Griffin JP. Drug interactions with antimalarial agents. Adverse Drug React Toxicol Rev. 1999; 18: 25–43.
- Segelnick SL, Weinberg MA. Recognizing doxycycline-induced esophageal ulcers in dental practice: a case report and review. J Am Dent Assoc. 2008; 139:581–5.
Chemical Properties
Yellow Crystalline powder
Uses
Doxycycline hydrochloride is a salt prepared from doxycycline taking advantage of the basic dimethylamino group which protonates and readily forms a salt in hydrochloric acid solutions. The hydrochloride is the preferred formulation for pharmaceutical applications. Like all tetracyclines, doxycycline shows broad spectrum antibacterial and antiprotozoan activity and acts by binding to the 30S and 50S ribosomal subunits, blocking protein synthesis.
Uses
antibiotic
Uses
Doxycycline hydrochloride, is used to eliminate Borrelia burgdorferi and Anaplasma phagocytophilum in rodent reservoirs and to eliminate Ixodes scapularis ticks. It is a broad spectrum inhibitor used to inhibit matrix metalloproteinases (MMP), such as type 1 collagenase in studies on wound healing and tissue remodeling.
Definition
ChEBI: The hydrochloride salt of doxycycline.
Doxycycline hydrochloride Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Guanlang Biotechnology Co,.LTD | +8619930503252 | daisy@crovellbio.com | China | 5964 | 58 |
| Henan Suikang Pharmaceutical Co.,Ltd. | +8618239973690 | sales@suikangpharm.com | China | 179 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12457 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659; +8618596095638 | sales@sdperfect.com | China | 294 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-13474506593 +86-13474506593 | sarah@tnjone.com | China | 864 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
Related articles
- Synthesis, Detection and Application of Doxycycline Hydrochloride
- The drug form of doxycycline commonly used clinically is its hydrochloride (Doxycycline Hyclate), which is crystalline powder ....
- Sep 9,2022
View Lastest Price from Doxycycline hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-23 | Doxycycline hydrochloride
10592-13-9
|
US $88.00-80.00 / kg | 100kg | 99.99% | 100Tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-20 | Doxycycline HCL
10592-13-9
|
US $0.00 / kg | 1kg | 99% | 10000kg | Shaanxi TNJONE Pharmaceutical Co., Ltd | |
 |
2024-04-16 | Doxycycline HCL soluble powder
10592-13-9
|
US $0.00-0.00 / kg | 1kg | 10% | 100 | Hebei ZB Gamay Biological Technology Co.,Ltd |
-

- Doxycycline hydrochloride
10592-13-9
- US $88.00-80.00 / kg
- 99.99%
- Hebei Dangtong Import and export Co LTD
-

- Doxycycline HCL
10592-13-9
- US $0.00 / kg
- 99%
- Shaanxi TNJONE Pharmaceutical Co., Ltd
-

- Doxycycline HCL soluble powder
10592-13-9
- US $0.00-0.00 / kg
- 10%
- Hebei ZB Gamay Biological Technology Co.,Ltd
10592-13-9(Doxycycline hydrochloride)Related Search:
1of4