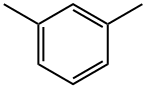
m-Xylene
- CAS No.
- 108-38-3
- Chemical Name:
- m-Xylene
- Synonyms
- 1,3-Xylene;1,3-DIMETHYLBENZENE;3-xylene;m-Xylol;META-XYLENE;Benzene,1,3-dimethyl-;2,4-Xylene;3-methyltoluene;m-XyL;M-XYLENE
- CBNumber:
- CB4852746
- Molecular Formula:
- C8H10
- Molecular Weight:
- 106.17
- MDL Number:
- MFCD00008536
- MOL File:
- 108-38-3.mol
- MSDS File:
- SDS
| Melting point | -48 °C (lit.) |
|---|---|
| Boiling point | 138-139 °C (lit.) |
| Density | 0.868 g/mL at 25 °C (lit.) |
| vapor density | 3.7 (vs air) |
| vapor pressure | 16 mm Hg ( 37.7 °C) |
| refractive index |
n |
| Flash point | 77 °F |
| storage temp. | 2-8°C |
| solubility | Miscible with many organic solvents, including alcohol and ether |
| form | Liquid |
| pka | >15 (Christensen et al., 1975) |
| color | Colorless |
| Odor | Like benzene; characteristic aromatic. |
| Odor Threshold | 0.041ppm |
| explosive limit | 1.1-7%(V) |
| Viscosity | 0.75mm2/s |
| Water Solubility | Miscible with organic solvents. Immiscible with water. |
| Merck | 14,10081 |
| BRN | 605441 |
| Henry's Law Constant | 14.80 at 45.00 °C, 17.26.4 at 50.00 °C, 19.90 at 55.00 °C, 23.01.3 at 60.00 °C, 27.46.3 at 70.00 °C (static headspace-GC, Park et al., 2004) |
| Exposure limits | NIOSH REL: 100 ppm (435 mg/m3), STEL 150 ppm (655 mg/m3), IDLH 900 ppm; OSHA PEL: TWA 100 ppm; ACGIH TLV: TWA 100 ppm, STEL 150 ppm (adopted). |
| Dielectric constant | 2.3999999999999999 |
| InChIKey | IVSZLXZYQVIEFR-UHFFFAOYSA-N |
| LogP | 3.16 at 20℃ |
| CAS DataBase Reference | 108-38-3(CAS DataBase Reference) |
| EWG's Food Scores | 7 |
| FDA UNII | O9XS864HTE |
| NIST Chemistry Reference | Benzene, 1,3-dimethyl-(108-38-3) |
| EPA Substance Registry System | m-Xylene (108-38-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H226-H304-H312+H332-H315-H319-H335-H412 | |||||||||
| Precautionary statements | P210-P273-P280-P301+P310-P303+P361+P353-P331 | |||||||||
| Hazard Codes | Xn,T,F | |||||||||
| Risk Statements | 10-20/21-38-39/23/24/25-23/24/25-11-36/38 | |||||||||
| Safety Statements | 25-45-36/37-16-7 | |||||||||
| RIDADR | UN 1307 3/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | ZE2275000 | |||||||||
| Autoignition Temperature | 982 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29024200 | |||||||||
| Toxicity | LD50 orally in rats: 7.71 ml/kg (Smyth) | |||||||||
| NFPA 704 |
|
m-Xylene price More Price(34)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.22337 | m-Xylene for synthesis | 108-38-3 | 1L | $108 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.22337 | m-Xylene for synthesis | 108-38-3 | 2.5L | $226 | 2024-03-01 | Buy |
| Sigma-Aldrich | 185566 | m-Xylene ReagentPlus , 99% | 108-38-3 | 1l | $204 | 2024-03-01 | Buy |
| Sigma-Aldrich | 185566 | m-Xylene ReagentPlus , 99% | 108-38-3 | 4l | $501 | 2024-03-01 | Buy |
| Sigma-Aldrich | 40202 | m-Xylene solution certified reference material, 5000?μg/mL in methanol | 108-38-3 | 1mL | $55.4 | 2022-05-15 | Buy |
m-Xylene Chemical Properties,Uses,Production
Description
Xylene exists in three isomeric forms, ortho-,meta-, and para-xylene. Commercial xylene is a mixture ofthese three isomers and may also contain ethylbenzene aswell as small amounts of toluene, trimethylbenzene, phenol,thiophene, pyridine, and other nonaromatic hydrocarbons.m-Xylene is predominant in commercial xylene. The physical properties of the three isomers are as follows:Isomer MeltingPoint(℃)BoilingPoint(℃)FlashPoint(℃)LowerExpl.(%)UpperExpl.(%)AutoTemp.(℃)ortho- -25 144 32 0.9 6.7 463meta- -48 139 27 1.1 7.0 527para- 13 138 27 1.1 7.0 528
Chemical Properties
clear colourless liquid
Chemical Properties
Xylene exists in three isomeric forms, ortho-, meta-, and para-xylene. Commercial xylene is a mixture of these three isomers and may also contain ethylbenzene as well as small amounts of toluene, trimethylbenzene, phenol, thiophene, pyridine, and other nonaromatic hydrocarbons. m-Xylene is predominant in commercial xylene.
Physical properties
Clear, colorless, watery liquid with a sweet, aromatic odor. An odor threshold concentration of 48 ppbv was reported by Nagata and Takeuchi (1990).
Uses
Meta-Xylene is used for the production of isophthalic acid, of agriculture chemicals as pharmaceuticals. It finds applications in paint and varnish removers and aerosol paint concentrates. Product Data Sheet
Uses
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
Uses
m-Xylene is used in the production of isophthalic acid, which is a monomer and utilized in preparation of polyethylene terephthalate. It is used as an important starting material in the production of 2,4- and 2,6-xylidine. It is used as solvent in histology.
Definition
ChEBI: A xylene carrying methyl groups at positions 1 and 3.
Synthesis Reference(s)
Journal of the American Chemical Society, 79, p. 2910, 1957 DOI: 10.1021/ja01568a059
General Description
A colorless watery liquid with a sweet odor. Less dense than water. Insoluble in water. Irritating vapor.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
m-Xylene may react with oxidizing materials. .
Health Hazard
Vapors cause headache and dizziness. Liquid irritates eyes and skin. If taken into lungs, causes severe coughing, distress, and rapidly developing pulmonary edema. If ingested, causes nausea, vomiting, cramps, headache, and coma; can be fatal. Kidney and liver damage can occur.
Fire Hazard
Behavior in Fire: Vapor is heavier than air and may travel considerable distance to a source of ignition and flash back.
Flammability and Explosibility
Flammable
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Moderately toxic by intraperitoneal route. Wdly toxic by ingestion, skin contact, and inhalation. An experimental teratogen. Human systemic effects by inhalation: motor activity changes, ataxia, and irritabihty. Experimental reproductive effects. A severe skin irritant. A common air contaminant. A very dangerous fire hazard when exposed to heat or flame; can react with oxidzing materials. Explosive in the form of vapor when exposed to heat or flame. To fight fire, use foam, CO2, dry chemical. Emitted from modern building materials (CENEAR 69,22,91). When heated to decomposition it emits acrid smoke and irritating fumes. See also other xylene entries.
Potential Exposure
Xylene is used as a solvent; as a constituent of paint, lacquers, varnishes, inks, dyes, adhesives, cements, cleaning fluids, and aviation fuels; and as a chemical feed-stock for xylidines, benzoic acid; phthalic anhydride; isophthalic, and terephthalic acids; as well as their esters (which are specifically used in the manufacture of plastic materials and synthetic textile fabrics). Xylene is also used in the manufacture of quartz crystal oscillators, hydrogen peroxide; perfumes, insect repellants; epoxy resins; pharmaceuticals; and in the leather industry. m-Xylene is used as an intermediate in preparation of isophthalic acid; o-xylene is used in manufacture of phthalic anhydride and in pharmaceutical and insecticide synthesis. p-xylene is used in pharmaceutical and insecticide synthesis and in production of polyester.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately.If this chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medicalfacility. If victim is conscious, administer water or milk. Donot induce vomiting.Note to physician: May require supportive measures forpulmonary edema.
Source
As m+p-xylene, detected in distilled water-soluble fractions of 87 octane gasoline, 94
octane gasoline, and Gasohol at concentrations of 7.00, 20.1, and 14.6 mg/L, respectively (Potter,
1996); in distilled water-soluble fractions of new and used motor oil at concentrations of 0.26 to
0.29 and 302 to 339 μg/L, respectively (Chen et al., 1994). The average volume percent and
estimated mole fraction in American Petroleum Institute PS-6 gasoline are 4.072 and 0.04406,
respectively (Poulsen et al., 1992). Diesel fuel obtained from a service station in Schlieren,
Switzerland contained m/p-xylene at a concentration of 336 mg/L (Schluep et al., 2001).
Thomas and Delfino (1991) equilibrated contaminant-free groundwater collected from
Gainesville, FL with individual fractions of three individual petroleum products at 24–25 °C for
24 h. The aqueous phase was analyzed for organic compounds via U.S. EPA approved test method
602. Average m+p-xylene concentrations reported in water-soluble fractions of unleaded gasoline,
kerosene, and diesel fuel were 8.611, 0.658, and 0.228 mg/L, respectively. When the authors
analyzed the aqueous-phase via U.S. EPA approved test method 610, average m+p-xylene
concentrations in water-soluble fractions of unleaded gasoline, kerosene, and diesel fuel were
lower, i.e., 6.068, 0.360, and 0.222 mg/L, respectively.
Based on laboratory analysis of 7 coal tar samples, m+p-xylene concentrations ranged from ND
to 6,000 ppm (EPRI, 1990). A high-temperature coal tar contained m-xylene at an average
concentration of 0.07 wt % (McNeil, 1983).
Schauer et al. (2001) measured organic compound emission rates for volatile organic
compounds, gas-phase semi-volatile organic compounds, and particle-phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The gas-phase emission
rate of m-xylene + p-xylene was 60.0 mg/kg of pine burned. Emission rates of both isomers were
not measured during the combustion of oak and eucalyptus.
Drinking water standard (final): For all xylenes, the MCLG and MCL are both 10 mg/L. In
addition, a DWEL of 70 mg/L was recommended (U.S. EPA, 2000).
Environmental Fate
Biological. Microbial degradation produced 3-methylbenzyl alcohol, 3-methylbenzaldehyde, mtoluic
acid, and 3-methylcatechol (quoted, Verschueren, 1983). m-Toluic acid was reported to be
the biooxidation product of m-xylene by Nocardia corallina V-49 using n-hexadecane as the
substrate (Keck et al., 1989). Reported biodegradation products of the commercial product
containing xylene include α-hydroxy-p-toluic acid, p-methylbenzyl alcohol, benzyl alcohol, 4-
methylcatechol, m- and p-toluic acids (Fishbein, 1985). In anoxic groundwater near Bemidji, MI,
m-xylene anaerobically biodegraded to the intermediate m-toluic acid (Cozzarelli et al., 1990). In
gasoline-contaminated groundwater, methylbenzylsuccinic acid was identified as the first
intermediate during the anaerobic degradation of xylenes (Reusser and Field, 2002).
Photolytic. When synthetic air containing gaseous nitrous acid and m-xylene was exposed to
artificial sunlight (λ = 300–450 nm) biacetyl, peroxyacetal nitrate, and methyl nitrate were formed
(Cox et al., 1980). They reported a rate constant of 1.86 x 10-11 cm3/molecule?sec for the reaction
of gaseous m-xylene with OH radicals based on a value of 8 x 10-12 cm3/molecule?sec for the
reaction of ethylene with OH radicals.
Chemical/Physical. Under atmospheric conditions, the gas-phase reaction with OH radicals and
nitrogen oxides resulted in the formation of m-tolualdehyde, m-methylbenzyl nitrate, nitro- mxylenes,
2,4- and 2,6-dimethylphenol (Atkinson, 1990). Kanno et al. (1982) studied the aqueous
reaction of m-xylene and other aromatic hydrocarbons (benzene, toluene, o- and p-xylene, and
naphthalene) with hypochlorous acid in the presence of ammonium ion. They reported that the
aromatic ring was not chlorinated as expected but was cleaved by chloramine forming cyanogen
chloride. The amount of cyanogen chloride formed increased at lower pHs (Kanno et al., 1982). In
the gas phase, m-xylene reacted with nitrate radicals in purified air forming pmethylbenzaldehyde,
an aryl nitrate and trace amounts of 2,6-dimethylnitrobenzene, 2,4-
dimethylnitrobenzene, and 3,5-dimethylnitrobenzene (Chiodini et al., 1993).
storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with this chemical you should be trainedon its proper handling and storage. Xylenes must be storedto avoid contact with strong oxidizers (such as chlorine,bromine, and fluorine) since violent reactions occur.Sources of ignition, such as smoking and open flames, areprohibited where xylenes are used, handled, or stored in amanner that could create a potential fire or explosionhazard. Use only nonsparking tools and equipment, especially when opening and closing containers of xylenes.Protect storage containers from physical damage.
Shipping
UN1307 Xylenes, Hazard Class: 3; Labels: 3-Flammable liquid.
Purification Methods
The general purification methods listed under xylene are applicable. The o-and p-isomers can be removed by their selective oxidation when a m-xylene sample containing them is boiled with dilute HNO3 (one part conc acid to three parts water). After washing with water and alkali, the product can be steam distilled, collected as for o-xylene, then distilled and purified further by sulfonation. [Clarke & Taylor J Am Chem Soc 45 831 1923.] m-Xylene is selectively sulfonated when a mixture of xylenes is refluxed with the theoretical amount of 50-70% H2SO4 at 85-95o under reduced pressure. By using a still resembling a Dean and Stark apparatus, water in the condensate can be progressively withdrawn while the xylene is returned to the reaction vessel. After cooling, then adding water, unreacted xylenes are distilled off under reduced pressure. The m-xylene sulfonic acid is subsequently hydrolysed by steam distillation up to 140o, the free m-xylene is washed, dried with silica gel and again distilled. It is stored over molecular sieves Linde type 4A. [Beilstein 5 H 370, 5 I 182, 5 II 287, 5 III 823, 5 IV 932.]
Incompatibilities
Vapor may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides. Electrostatic charges can be generated from agitation or flow.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration.
m-Xylene Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of7
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7378 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 | sales@finerchem.com | China | 2966 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
View Lastest Price from m-Xylene manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-17 | 1,3-Dimethylbenzene
108-38-3
|
US $5.00 / kg | 1kg | ≥98% | 200mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2023-12-26 | m-Xylene
108-38-3
|
US $100.00-1.00 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2023-08-24 | m-Xylene
108-38-3
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd |
-

- 1,3-Dimethylbenzene
108-38-3
- US $5.00 / kg
- ≥98%
- Jinan Finer Chemical Co., Ltd
-

- m-Xylene
108-38-3
- US $100.00-1.00 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- m-Xylene
108-38-3
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
108-38-3(m-Xylene)Related Search:
1of4