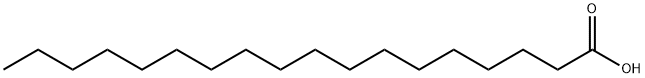
Stearic acid
- CAS No.
- 57-11-4
- Chemical Name:
- Stearic acid
- Synonyms
- OCTADECANE;Octadecanoic acid;C18;OCTADECANEDIOIC ACID;C18:0;Stearic;Steric acid;TRIPLE PRESSED STEARIC ACID;1800;Stearil Acid
- CBNumber:
- CB4853859
- Molecular Formula:
- C18H36O2
Lewis structure
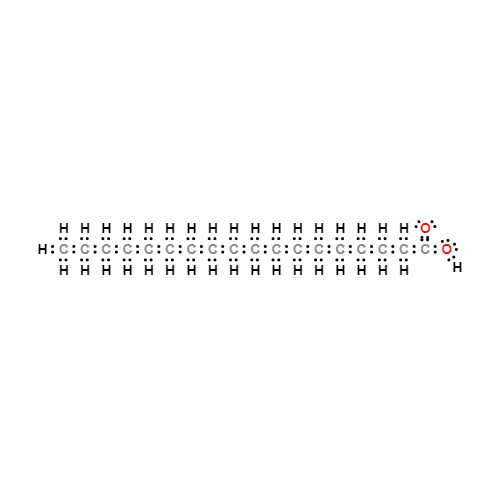
- Molecular Weight:
- 284.48
- MDL Number:
- MFCD00002752
- MOL File:
- 57-11-4.mol
- MSDS File:
- SDS
| Melting point | 67-72 °C (lit.) |
|---|---|
| Boiling point | 361 °C (lit.) |
| Density | 0.845 g/cm3 |
| vapor pressure | 1 mm Hg ( 173.7 °C) |
| FEMA | 3035 | STEARIC ACID |
| refractive index | 1.4299 |
| Flash point | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | Practically insoluble in water, soluble in ethanol (96 per cent) and in light petroleum (bp: 50-70 °C). |
| form | powder |
| pka | pKa 5.75±0.00(H2O t = 35) (Uncertain) |
| Specific Gravity | 0.84 (80℃) |
| color | White |
| Odor | odorless mild fatty |
| Odor Type | odorless |
| Water Solubility | 0.1-1 g/100 mL at 23 ºC |
| JECFA Number | 116 |
| Merck | 14,8804 |
| BRN | 608585 |
| Dielectric constant | 2.3(22℃) |
| Exposure limits | ACGIH: TWA 10 mg/m3; TWA 3 mg/m3 |
| InChIKey | QIQXTHQIDYTFRH-UHFFFAOYSA-N |
| LogP | 8.22 |
| FDA 21 CFR | 184.1090; 172.615; 175.105; 175.300; 201.327; 352.70; 357.210 |
| Substances Added to Food (formerly EAFUS) | STEARIC ACID |
| SCOGS (Select Committee on GRAS Substances) | Stearic acid (packaging) |
| CAS DataBase Reference | 57-11-4(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 4ELV7Z65AP |
| NIST Chemistry Reference | Octadecanoic acid(57-11-4) |
| EPA Substance Registry System | Stearic acid (57-11-4) |
| Cosmetics Info | Stearic Acid |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P280a-P304+P340-P305+P351+P338-P405-P501a | |||||||||
| Hazard Codes | Xi,F | |||||||||
| Risk Statements | 38-36/37/38-11 | |||||||||
| Safety Statements | 37/39-26-16 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | WI2800000 | |||||||||
| Autoignition Temperature | 395 °C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 38231100 | |||||||||
| Toxicity | LD50 i.v. in mice, rats: 23±0.7, 21.5±1.8 mg/kg, L. Or, A. Wretlind, Acta Pharmacol. Toxicol. 18, 141 (1961) | |||||||||
| NFPA 704 |
|
Stearic acid price More Price(71)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W303518 | Stearic acid ≥95%, FCC, FG | 57-11-4 | 1kg | $76.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | W303518 | Stearic acid ≥95%, FCC, FG | 57-11-4 | 8kg | $244 | 2024-03-01 | Buy |
| Sigma-Aldrich | W303518 | Stearic acid ≥95%, FCC, FG | 57-11-4 | 25kg | $328 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.00673 | Stearic acid for synthesis | 57-11-4 | 500g | $60.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.00673 | Stearic acid for synthesis | 57-11-4 | 1kg | $86.5 | 2024-03-01 | Buy |
Stearic acid Chemical Properties,Uses,Production
description
Stearic acid is one of several major long-chain fatty acids comprising oils and fats. It is presented in animal fats, oil and some kinds of vegetable oils as wellin the form of glycerides. These oils, after hydrolysis, produce the stearic acid.
Stearic acid is a fatty acid widely existing in nature and has the general chemical properties of carboxylic acids. Almost all kinds of fat and oil contain certain amount of stearic acid with the content in the animal fats being relative high. For example, the content in the butter can reach up to 24% while the content in vegetable oil is relative low with the value in tea oil being 0.8% and the oil in palm being 6%. However, the content in cocoa can reach as high as 34%.
There are two major approaches for industrial production of stearic acid, namely fractionation and compression method. Add decomposition agent to the hydrogenated oil, and then hydrolyze to give the crude fatty acid, further go through washing with water, distillation, bleaching to obtain the finished products with glycerol as the byproduct.
Most domestic manufacturers use animal fat for production. Some kinds of production technology will result in the incompletion of the distillation of fatty acid which produce stimulating odor at the time of the plastic processing and high temperatures. Although these odor is of no toxic but they will have certain effect on the working conditions and the natural environment. Most imported form of stearic acid takes vegetable oil as the raw materials, the production processes are more advanced; the produced stearic acid is of stable performance, good lubrication property and less odor in the application.
Stearic acid is mainly used for the production of stearates such as sodium stearate, magnesium stearate, calcium stearate, lead stearate, aluminum stearate, cadmium stearate, iron stearate, and potassium stearate. The sodium or potassium salt of stearic acid is the component of soap. Although sodium stearate has a less decontamination ability than sodium palmitate, but its presence may increase the hardness of soap.
Take butter as raw material, go through sulfuric acid or pressurized method for decomposition. The free fatty acids was first subject to water pressure method for removing the palmitic acid and oleic acid at 30~40 ℃, and then dissolved in ethanol, followed by addition of barium acetate or magnesium acetate which precipitates stearate. Then further add dilute sulfuric acid to get the free stearate acid, filter and take it, and re-crystallize in ethanol to obtain the pure stearic acid.
The above information is edited by the chemicalbook of Dai Xiongfeng.
Chemical Properties
Pure product appears as white shiny soft small pieces. It is slightly soluble in water, soluble in alcohol, acetone, easily soluble in benzene, chloroform, ether, carbon tetrachloride, carbon disulfide, amyl acetate and toluene.
application
Stearic acid is widely used in cosmetics, plastics plasticizers, mold release agents, stabilizers, surfactants, rubber vulcanization accelerator, waterproof agent, polishing agent, metal soap, metal mineral flotation agents, softeners and pharmaceuticals as well as other organic chemicals. Stearic acid can also be used as the solvents of oil-soluble paint, crayons lubrication agent, stencil lighting agent and the emulsifier of stearic acid glyceride.
Stearic acid can also be widely used in the manufacturing of PVC pipe, sheet material, profiles and film and is the PVC heat stabilizers with good lubricity and excellent stability against light and heat. In the application of polyvinyl chloride pipe, stearic acid helps prevent the "coke" during the processing and is effective heat stabilizer during PVC film processing while also preventing the discoloration of the finished film discoloration caused by exposure.
Stearic acid has become the additive for lubrication, plasticization and stabilization of the filled masterbatch. Stearic acid can effectively improve the coating activating effect of inorganic powder and increase the flow rate of materials. When there is demand for a large flow rate of the melt for material with inorganic powder accounting for the most part, an appropriate increase in the content of stearic acid can significantly increase the melt flow rate of material. However, the amount of stearic acid used in filled masterbatch also have threshold with its amount being controlled in about 1% of the total mass. If the added amount is over-excessive, it will not only cause the decrease of the quality and the performance of plastic products but also generate sticky substance in the die lip location of the manufacturing equipment of the plastic products, affecting the production efficiency and product quality.
The mono-or multi-alcohol ester of stearic acid can be used as cosmetics, nonionic surfactants and plasticizers. Its alkali metal salt can be dissolved in water and is a major component of soap. Other kinds of salts can be used as waterproofing agents, lubricants, bactericides, coating additives and PVC stabilizers.
Uses
It can be used as natural rubber, synthetic rubber (except butyl rubber) and latex curing active agent. It can also be used as raw material of plastic plasticizer and stabilizer. Medicine: it can be used for the preparation of ointments, suppositories, etc., as well as being used in the manufacture of cosmetics, candles, waterproof agent and polishing agent. The product can be used as a lubricant, defoamers and food additives in the food industry as well as the raw materials of glycerol stearate, stearic acid sorbitol anhydride esters and sucrose esters.
It can also be used as standard reference product for gas analysis as well as the preparation of soap, cosmetics, pharmaceuticals and other organic chemicals.
Toxicity
LD50 i.v. in mice, rats: 23±0.7, 21.5±1.8 mg/kg, L. Or, A. Wretlind, Acta Pharmacol. Toxicol. 18, 141 (1961)
Limited use
FEMA (mg/kg): soft drinks: 2.0~ 10; candy: 4000; bakery: 3.5.
GB 2760-2001: candy, gum base agent; take GMP as limit.
Production method
There are two major approaches for industrial production of stearic acid, namely fractionation and compression method. Add decomposition agent to the hydrogenated oil, and then hydrolyze to give the crude fatty acid, further go through washing with water, distillation, bleaching to obtain the finished products with glycerol as the byproduct.
Compression method takes animal oil as raw material. Have animal oil subject to hydrolysis in the catalysis of zinc oxide at pressure of 1.17~1.47 MPa, further go through pickling, washing, distillation, cooling, freezing, press for removal of oleic acid to get the finished products.
Heat the cotton seed oil, rice bran oil, or soybean oil in the presence of a hydrolyzing agent under normal pressure to boiling with hydrolysis of 1.5 h and harden to saturated fatty acid. Oleic acid hydrogenation;
Use the C10~C20 and C18~C20 fraction of the synthetic fatty acid as raw materials, go through melting, pickling (with 1% sulfuric acid) mold, pressing, melting, pickling, dehydrating and crystallization to obtain it.
It can be obtained through the low-temperature segment separation of the mixed fatty acid.
It can also be made through the hydrogenation of oleic acid.
Description
Stearic acid is a long-chain saturated fatty acid. It is a major component of cocoa butter and has also been found in beef fat and vegetable oils. Unlike many long-chain saturated fatty acids, dietary stearic acid does not induce hypercholesterolemia or raise LDL-cholesterol.
Description
Stearic acid (STAIR-ik or STEER-ik) is the saturated fatty acid with an 18 carbon chain and has the IUPAC name octadecanoic acid. It is a waxy solid, and its chemical formula is CH3(CH2)16CO2H. Its name comes from the Greek word στ?αρ "stéar", which means tallow. The salts and esters of stearic acid are called stearates. Stearic acid is one of the most common saturated fatty acids found in nature following palmitic acid.
Chemical Properties
Stearic acid has a characteristic odor and taste resembling tallow. It is a mixture of solid organic acids obtained from fats consisting chiefly of stearic acid (C18H36O2) and palmitic acid (C16H32O2).
Chemical Properties
Stearic acid is a hard, white or faintly yellow-colored, somewhat glossy, crystalline solid or a white or yellowish white powder. It has a slight odor (with an odor threshold of 20 ppm) and taste suggesting tallow.
Chemical Properties
Stearic acid, CH3(CH2)16COOH, is a white or colorless, waxlike solid with a melting point of 70°C (158 OF), and a boiling point of 232°C (450 OF) at 2 kPa. It is soluble in alcohol, ether, and chloroform,and is insolublein water. Stearic acid, nature's most common fatty acid, is derived from natural animal and vegetable fats. Also known as n-octadecanoic acid, stearic acid is used in the preparation of metallic stearates, as a lubricant, and in pharmaceuticals, cosmetics, candles, and food packaging.
Occurrence
Stearic acid is naturally present in the glycerides of animal fats and most vegetable oils. Reported found in fresh apple, banana, Vitis vinifera L., melon, tomato, ginger, blue cheeses, cheddar cheese, Swiss cheese, feta cheese, buttermilk, raw fatty fish, raw lean fish, raw shrimp, grapefruit juice, guava, papaya, cucumber, saffron, pork and lamb liver, pork fat, hop oil, beer, cognac, rum, whiskies, sherry, tea, peanut oil, soybean, roast coconut, coconut milk, avocado, passion fruit, rose apple, mushroom, starfruit, fenugreek, mango, cardamom, cooked rice, prickly pear, dill seed, buckwheat, malt, wort, cassava, loquat, shrimp, crab, cape gooseberry and Chinese quince.
Uses
Generally applications of stearic acid exploit its bifunctional character, with a polar head group that can be attached to metal cations and a nonpolar chain that confers solubility in organic solvents. The combination leads to uses as a surfactant and softening agent. Stearic acid undergoes the typical reactions of saturated carboxylic acids, notably reduction to stearyl alcohol, and esterification with a range of alcohols.
Soaps , cosmetics , detergents
Stearic acid is mainly used in the production of detergents, soaps, and cosmetics such as shampoos and shaving cream products. Soaps are not made directly from stearic acid, but indirectly by saponification of triglycerides consisting of stearic acid esters. Esters of stearic acid with ethylene glycol; glycol stearate and glycol distearate, are used to produce a pearly effect in shampoos, soaps, and other cosmetic products. They are added to the product in molten form and allowed to crystallize under controlled conditions. Detergents are obtained from amides and quaternary alkylammonium derivatives of stearic acid.
Lubricants , softening and release agents
In view of the soft texture of the sodium salt, which is the main component of soap, other salts are also useful for their lubricating properties. Lithium stearate is an important component of grease. The stearate salts of zinc, calcium, cadmium, and lead are used to soften PVC. Stearic acid is used along with castor oil for preparing softeners in textile sizing. They are heated and mixed with caustic potash or caustic soda. Related salts are also commonly used as release agents, e.g. in the production of automobile tires.
Niche uses
Being inexpensively available and chemically benign, stearic acid finds many niche applications. When making plaster castings from a plaster piece mold or waste mold and when making the mold from a shellacked clay original. In this use, powdered stearic acid is mixed in water and the suspension is brushed onto the surface to be parted after casting. This reacts with the calcium in the plaster to form a thin layer of calcium stearate which functions as a release agent. When reacted with zinc it forms zinc stearate which is used a lubricant for playing cards (fanning powder) to ensure a smooth motion when fanning. In compressed confections, it is used as a lubricant to keep the tablet from sticking to the die.
Fatty acids are classic components of candle - making. Stearic acid is used along with simple sugar or corn syrup as a hardener in candies.
Stearic acid is used to produce dietary supplements.
In fire works, stearic acid is often used to coat metal powders such as aluminium and iron. This prevents oxidation, allowing compositions to be stored for a longer period of time. Stearic acid is a common lubricant during injection molding and pressing of ceramic powders. It is also used as a mold release for foam latex that is baked in stone molds. .
Uses
Pharmaceutic aid (emulsion adjunct); pharmaceutic aid (tablet and/or capsule lubricant).
Uses
Stearic Acid is a fatty acid that is a mixture of solid organic acids obtained principally from stearic acid and palmitic acid. it is practi- cally insoluble in water. it functions as a lubricant, binder, and defoamer. it is used as a softener in chewing gum base.
Uses
stearic acid is an emulsifier and thickening agent found in many vegetable fats. Stearic acid is the main ingredient used in making bar soaps and lubricants. It occurs naturally in butter acids, tallow, cascarilla bark, and in other animal fats and oils. Stearic acid may cause allergic reactions in people with sensitive skin and is considered somewhat comedogenic.
Definition
ChEBI: A C18 straight-chain saturated fatty acid component of many animal and vegetable lipids. As well as in the diet, it is used in hardening soaps, softening plastics and in making cosmetics, candles and plastics.
Definition
A solid carboxylic acid present in fats and oils as the glyceride.
Production Methods
Stearic Acid occurs in many animal and vegetable fats and oils, but it is more abundant in animal fat (up to 30 %) than vegetable fat (typically < 5 % ) . The important exceptions are cocoa butter and shea butter where the stearic acid content (as a triglyceride) is 28 – 45 %.
Stearic acid is prepared by treating these fats and oils with water at a high pressure and temperature (above 200 °C), leading to the hydrolysis of triglycerides. The resulting mixture is then distilled. Commercial stearic acid is often a mixture of stearic and palmitic acids, although purified stearic acid is available.
In terms of its biosynthesis, stearic acid is produced from carbohydrates via the fatty acid synthesis machinery via acetyl-CoA .
Preparation
Commercially it is produced by the hydrogenation of the unsaturated 18-carbon fatty acids of soybean, cottonseed or other vegetable oils. When obtained from animal fats by hydrolysis and fractional crystallization, commercial stearic acid is a mixture of solid organic acids, chiefly palmitic and stearic acids. Commercial products containing about 90% stearic acid are produced by hydrolysis and crystallization of a completely hydrogenated vegetable oil or by fractional distillation of fatty acid mixtures obtained from tallow
Production Methods
Stearic acid is manufactured by hydrolysis of fat by continuous
exposure to a countercurrent stream of high-temperature water and
fat in a high-pressure chamber. The resultant mixture is purified by
vacuum steam distillation and the distillates are then separated
using selective solvents.
Stearic acid may also be manufactured by the hydrogenation of
cottonseed and other vegetable oils; by the hydrogenation and
subsequent saponification of olein followed by recrystallization
from alcohol; and from edible fats and oils by boiling with sodium
hydroxide, separating any glycerin, and decomposing the resulting
soap with sulfuric or hydrochloric acid. The stearic acid is then
subsequently separated from any oleic acid by cold expression.
Stearic acid is derived from edible fat sources unless it is intended
for external use, in which case nonedible fat sources may be used.
The USP32–NF27 states that stearic acid labeled solely for external
use is exempt from the requirement that it be prepared from edible
sources. Stearic acid may contain a suitable antioxidant such as
0.005% w/w butylated hydroxytoluene.
brand name
Hystrene 5016 (Witco).
Aroma threshold values
Detection: 20 ppm
Synthesis Reference(s)
Synthetic Communications, 15, p. 759, 1985 DOI: 10.1080/00397918508063869
General Description
White solid with a mild odor. Floats on water.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Stearic acid is incompatible with strong oxidizers and strong bases. Stearic acid is also incompatible with reducing agents.
Health Hazard
Compound is generally considered nontoxic. Inhalation of dust irritates nose and throat. Dust causes mild irritation of eyes.
Fire Hazard
Stearic acid is combustible. Stearic acid can heat spontaneously.
Pharmaceutical Applications
Stearic acid is widely used in oral and topical pharmaceutical
formulations. It is mainly used in oral formulations as a tablet and
capsule lubricant, although it may also be used as a
binder or in combination with shellac as a tablet coating. It has
also been suggested that stearic acid may be used in enteric tablet
coatings and as a sustained-release drug carrier.
In topical formulations, stearic acid is used as an emulsifying and
solubilizing agent. When partially neutralized with alkalis or
triethanolamine, stearic acid is used in the preparation of
creams. The partially neutralized stearic acid forms a creamy
base when mixed with 5–15 times its own weight of aqueous liquid,
the appearance and plasticity of the cream being determined by the
proportion of alkali used.
Stearic acid is used as the hardening agent in glycerin
suppositories.
Stearic acid is also widely used in cosmetics and food products.
Biochem/physiol Actions
β-Oxidation of stearic acid yields eight FADH2 (flavin adenine dinucleotide) and NADH2 (nicotinamide adenine dinucleotide) molecules and nine acetyl-CoA molecules.
Safety Profile
Poison by intravenous route. A human sktn irritant. Questionable carcinogen with experimental tumorigenic data by implantation route. Combustible when exposed to heat or flame. Heats spontaneously. To fight fire, use CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
Safety
Stearic acid is widely used in oral and topical pharmaceutical
formulations; it is also used in cosmetics and food products. Stearic
acid is generally regarded as a nontoxic and nonirritant material.
However, consumption of excessive amounts may be harmful.
LD50 (mouse, IV): 23 mg/kg
LD50 (rat, IV): 21.5 mg/kg
Carcinogenicity
Stearic acid was administered subcutaneously to several groups of Swiss–Webster mice at doses of 0.05 or 0.5mg once weekly for 25 weeks (total dose of 1.3–130 mg), 1.0 mg thrice a week for a total of 10 injections or 1.0 mg twice weekly for a total of 82 injections. No neoplasms were reported in these studies . In 3 groups of 10–15 BALB/c mice administered 0.05 mg or 0.5 mg stearic acid (twice weekly for 52 or 57 weeks), one pulmonary neoplasm was detected in each group after 19–21 months.Afewsubcutaneous sarcomas and one adrenal carcinoma were also reported.No injection site sarcomas or other carcinogenic effects were reported by the same authors in a later study of mice injected with 0.05–0.5 mg weekly for 26 weeks. Rats given subcutaneous injections of 0.05 or 0.5 mg stearic acid weekly for 26 weeks did not develop sarcomas at the site of injection. When rat fibroblast cells were transfected with an activated human c-H-ras oncogene and the cells subsequently grown in a medium supplemented with stearic acid (20–80 mM), there was a marked increase in the number of transformed foci. Stearic acid inhibited the colony-forming ability of four out of five rat and two human tumor continuous cell lines in vitro. Using rats pretreated with nitrosomethyl urea as a model for mammary carcinoma, Habib et al. demonstrated that subcutaneous injection of stearic acid at weekly intervals prevented tumor development. Increasing levels of stearate in the diet resulted in decreased mammary tumor incidence and increased time to tumor in mice.
Metabolism
An isotope labeling study in humans concluded that the fraction of dietary stearic acid oxidatively desaturated to oleic acid was 2.4 times higher than the fraction of palmitic acid analogously converted to palmitoleic acid. Also, stearic acid was less likely to be incorporated into cholesterol esters. In epidemiologic and clinical studies stearic acid was associated with lowered LDL cholesterol in comparison with other saturated fatty acids. These findings may indicate that stearic acid is healthier than other saturated fatty acids.
storage
Stearic acid is a stable material; an antioxidant may also be added to it. The bulk material should be stored in a wellclosed container in a cool, dry place.
Purification Methods
Crystallise stearic acid from acetone, acetonitrile, EtOH (5 times), aqueous MeOH, ethyl methyl ketone or pet ether (b 60-90o), or by fractional precipitation by dissolving in hot 95% EtOH and pouring into distilled water, with stirring. The precipitate, after washing with distilled water, is dried under vacuum over P2O5. It has also been purified by zone melting and partial freezing. [Tamai et al. J Phys Chem 91 541 1987, Beilstein 2 IV 1206.]
Incompatibilities
Stearic acid is incompatible with most metal hydroxides and may be
incompatible with bases, reducing agents, and oxidizing agents.
Ointment bases made with stearic acid may show evidence of
drying out or lumpiness due to such a reaction when compounded
with zinc or calcium salts.
A number of differential scanning calorimetry studies have
investigated the compatibility of stearic acid with drugs. Although
such laboratory studies have suggested incompatibilities, e.g. with
naproxen, they may not necessarily be applicable to formulated
products.
Stearic acid has been reported to cause pitting in the film coating
of tablets applied using an aqueous film-coating technique; the
pitting was found to be a function of the melting point of the stearic
acid.
Regulatory Status
GRAS listed. Accepted as a food additive in Europe (fatty acids). Included in the FDA Inactive Ingredients Database (sublingual tablets; oral capsules, solutions, suspensions, and tablets; topical and vaginal preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Stearic acid Preparation Products And Raw materials
Raw materials
1of4
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
| ShanDong Look Chemical Co.,Ltd. | +8617653113219 | sales01@sdlookchemical.com | China | 2739 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | qinhe02@xaltbio.com | China | 1000 | 58 |
| Hebei Xinsheng New Material Technology Co., LTD. | +86-16632316109 | xinshengkeji@xsmaterial.com | China | 1100 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 | hbjbtech@163.com | China | 1000 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7786 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-13474506593 +86-13474506593 | sarah@tnjone.com | China | 874 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 | catherine@yjchem.com.cn | China | 136 | 58 |
View Lastest Price from Stearic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-24 | Stearic acid
57-11-4
|
US $1250.00 / T | 0.1T | 98% | 20T | Yujiang Chemical (Shandong) Co.,Ltd. | |
 |
2024-04-24 | Stearic acid
57-11-4
|
US $1100.00-990.00 / metric tonnes | 1metric tonnes | 99% | 100tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-22 | Stearic acid
57-11-4
|
US $1500.00 / T | 1T | 99% | 1000tons | Hebei Yime New Material Technology Co., Ltd. |
-

- Stearic acid
57-11-4
- US $1250.00 / T
- 98%
- Yujiang Chemical (Shandong) Co.,Ltd.
-

- Stearic acid
57-11-4
- US $1100.00-990.00 / metric tonnes
- 99%
- Hebei Dangtong Import and export Co LTD
-

- Stearic acid
57-11-4
- US $1500.00 / T
- 99%
- Hebei Yime New Material Technology Co., Ltd.